Efficacy and Safety of Intravitreal Aflibercept Injection in Japanese Patients with Neovascular Glaucoma: Outcomes from the VENERA Study
- PMID: 33330959
- PMCID: PMC7889553
- DOI: 10.1007/s12325-020-01580-y
Efficacy and Safety of Intravitreal Aflibercept Injection in Japanese Patients with Neovascular Glaucoma: Outcomes from the VENERA Study
Abstract
Introduction: Neovascular glaucoma is characterized by neovascularization of the iris and anterior angle chamber. Intravitreal anti-vascular endothelial growth factor agents may decrease intraocular pressure (IOP) and improve neovascularization. The VENERA study assessed the efficacy and safety of intravitreal aflibercept (IVT-AFL) in patients with neovascular glaucoma.
Methods: This was a 5-week, single-arm, nonrandomized, open-label, phase 3 study performed at 7 study sites in Japan that enrolled Japanese patients with anterior segment neovascularization and IOP > 25 mmHg who had not undergone (within 30 days prior), nor were imminently scheduled to undergo (within 8 days following) intraocular surgeries, including panretinal photocoagulation (PRP). Patients received background therapy plus 2 mg IVT-AFL at baseline. Background therapy with systemic IOP-lowering drugs was prohibited for 3 days before day 1 and until IOP evaluation at week 1. The primary endpoint was the change in IOP from baseline to week 1 and the secondary endpoint was the proportion of patients with an improvement of ≥ 1 grade of neovascularization of the angle (NVA) from baseline to week 1.
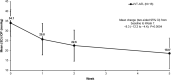
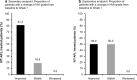
Results: Sixteen patients received treatment (full analysis set); the per-protocol set comprised 15 patients. The mean IOP decreased from 34.1 mmHg at baseline to 25.8 mmHg at week 1 (mean change, -8.3 mmHg [95% confidence interval; CI -12.2 to -4.4; P = 0.0004]). At week 1, 81.3% of patients had an improvement in the grade of neovascularization of the iris (NVI) and 50.0% of patients had an improvement in NVA grade. The proportion of patients with controlled IOP (≤ 21 mmHg) was 43.8% (95% CI 19.8-70.1) at week 1, and increased to 56.3% at week 2 and 86.7% at week 5. The most common ocular treatment-emergent adverse event was eye pain, which occurred in 4 patients (25.0%).
Conclusions: IVT-AFL was associated with statistically significant and clinically meaningful IOP reductions, without concomitant use of systemic IOP-lowering drugs or PRP. The safety profile was consistent with the known safety profile of IVT-AFL. These findings supplement those from the previous VEGA study, and suggest that IVT-AFL may be a potential treatment option for patients with neovascular glaucoma.
Trial registration: Clinicaltrials.gov identifier NCT03639675.
Keywords: Anti-VEGF; Anti-vascular endothelial growth factor; Intraocular pressure; Intravitreal aflibercept; NVG; Neovascular glaucoma; Neovascularization of the angle; Neovascularization of the iris.
Figures

References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

