Application of a hemophilia mortality framework to the Emicizumab Global Safety Database
- PMID: 33331041
- PMCID: PMC7756327
- DOI: 10.1111/jth.15187
Application of a hemophilia mortality framework to the Emicizumab Global Safety Database
Abstract
Background: As the first non-factor replacement therapy for persons with congenital hemophilia A (PwcHA), emicizumab's safety profile is of particular interest to the community.
Objectives: We applied an algorithm for categorization of fatal events contemporaneous to emicizumab using reporter-assessed causality documented in the Roche Emicizumab Global Safety Database.
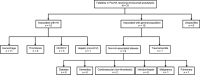
Patients/methods: All fatalities in PwcHA reported to the database (from clinical trials, pre-market access, and spontaneous post-marketing reports) were categorized into: associated with hemophilia A-hemorrhagic, thrombotic, human immunodeficiency virus (HIV)/hepatitis C virus (HCV), hepatic (non-HCV); associated with general population-trauma/suicide, non-HA-associated conditions; or, unspecified. Reported cause of death was not reassessed.
Results: As of cut-off May 15, 2020, 31 fatalities in PwcHA taking emicizumab were reported. Median age at death was 58 years; 51% had factor VIII inhibitors. Fifteen fatalities were considered associated with HA; overall, the most frequent category was hemorrhage (11/31). Of these, six had a history of life-threatening bleeds, and four had a history of intracranial hemorrhage. The remaining HA-associated fatalities were related to HIV/HCV (3/31) and other hepatic causes (1/31). No cases were categorized as thrombotic. Of 10 cases considered not associated with HA, two were categorized as cardiovascular (non-thrombotic), five as infection/sepsis, and one each of trauma/suicide, pulmonary, and malignancy. Six cases were unspecified.
Conclusions: No unique risk of death was associated with emicizumab prophylaxis in PwcHA. The data reveal that mortality in PwcHA receiving emicizumab was primarily associated with hemorrhage or non-HA-associated conditions, and was not reported by treaters to be related to emicizumab treatment.
Keywords: benchmarking; cause of death; hemophilia A; mortality; safety.
© 2020 The Authors. Journal of Thrombosis and Haemostasis published by Wiley Periodicals LLC on behalf of International Society on Thrombosis and Haemostasis.
Conflict of interest statement
None of the authors received honoraria or fees for their contribution to the development of this article or supplement. P.K. is a current employee of and holds shares in Genentech, Inc. Outside of this manuscript, most authors received fees for participation in activities funded by F. Hoffmann‐La Roche Ltd and/or Genentech, Inc., as described in the following disclosures, with the exception of G.F.P.
F.P. has received honoraria for participating as a speaker at satellite symposia organized by F. Hoffmann‐La Roche Ltd, Sanofi, Sobi, Spark, and Takeda, and has participated in advisory boards for F. Hoffmann‐La Roche Ltd, Sanofi, and Sobi. J.N.M. has received consultancy from CSL Behring, Catalyst Biosciences, Freeline Therapeutics, Novo Nordisk, F. Hoffmann‐La Roche Ltd, Sanofi, Spark, and Takeda, has received research funding from BioMarin, CSL Behring, Freeline Therapeutics, Novo Nordisk, Novartis, Pfizer, Sanofi, F. Hoffmann‐La Roche Ltd, and uniQure; has participated in a speakers’ bureau for CSL Behring, Catalyst Biosciences, Novo Nordisk, F. Hoffmann‐La Roche Ltd, Sanofi, Spark, and Takeda; and sits on the board of directors/advisory committee for the South Africa Medical Research Council, the Wits Health Consortium, and Colleges of Medicine of South Africa. S.W.P. has received consultancy for Apcintex, Bayer, BioMarin, Catalyst Biosciences, CSL Behring, HEMA Biologics, Freeline Therapeutics, Novo Nordisk, Pfizer, F. Hoffmann‐La Roche Ltd/Genentech, Inc., Sangamo Therapeutics, Sanofi, Takeda, Spark Therapeutics, and uniQure; has received research funding from Siemens; and is a member of the board of directors/advisory committee for the Medical and Scientific Advisory Council to the National Hemophilia Foundation and the Medical Advisory Board to World Federation of Hemophilia. C.R.M.H. has received consultancy and honoraria from Shire, Alnylam, Novo Nordisk, Sobi, and F. Hoffmann‐La Roche Ltd; has participated in speakers’ bureaus for Shire, Sobi, F. Hoffmann‐La Roche Ltd, and Pfizer; has received research funding from Bayer, Shire, Sobi, Novo Nordisk, and Pfizer; and has received travel and accommodation expenses from F. Hoffmann‐La Roche Ltd, Sobi, CSL Behring, and Shire. G.F.P. is a leader of WFH and NHF; has received honoraria from Griffols; has received fees for consultancy/advice for BioMarin, Takeda, Pfizer, Geneception, Generation Bio, CRISPR Therapeutics, and Third Rock Ventures; and has patent fees from Sanofi. P.K. is a current employee of and holds shares in Genentech, Inc. R.K.‐J. has received consultancy from Chugai Pharmaceutical Co., BioMarin, CSL Behring, CRISPR Therapeutics, Genentech, Inc., has received research funding from CSL Behring, Genentech, Inc., and Spark; has received honoraria from Chugai Pharmaceutical Co., BioMarin, CSL Behring, CRISPR Therapeutics, and Genentech, Inc., and has participated in speakers’ bureaus for F. Hoffmann‐La Roche Ltd. M.S. has received consultancy and honoraria from Chugai Pharmaceutical Co.; has received research funding from Chugai Pharmaceutical Co., F. Hoffmann‐La Roche Ltd, Sanofi, CSL Behring, KM Biologics, Novo Nordisk, Shire/Takeda; holds patents related to anti‐FIXa/FX bispecific antibodies; has participated in speakers’ bureaus for Chugai Pharmaceutical Co., Sanofi, Bayer, and Sysmex; and is a member of the board of directors/advisory committee for Chugai Pharmaceutical Co., F. Hoffmann‐La Roche Ltd, BioMarin, Bayer, and Sanofi.
Figures

References
-
- Reyes A, Révil C, Niggli M, et al. Efficacy of emicizumab prophylaxis versus factor VIII prophylaxis for treatment of hemophilia A without inhibitors: network meta‐analysis and sub‐group analyses of the intra‐patient comparison of the HAVEN 3 trial. Curr Med Res Opin. 2019;35:2079‐2087. - PubMed
-
- Pierce GF, Hart DP, Kaczmarek R. Safety and efficacy of emicizumab and other novel agents in newborns and infants. Haemophilia. 2019;25:e334‐e335. - PubMed
-
- Food and Drug Administration . HEMLIBRA® (emicizumab‐kxwh) injection for subcutaneous use, prescribing information. Initial U.S. approval: 2017. 2018; https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761083s000lbl.pdf. Accessed April 28, 2020.
-
- European Medicines Agency . HEMLIBRA® solution for injection: emicizumab piIEa. Initial EU approval: 2018. 2019. https://www.ema.europa.eu/en/documents/product‐information/hemlibra‐epar.... Accessed April 28, 2020.
-
- Kitazawa T, Igawa T, Sampei Z, et al. A bispecific antibody to factors IXa and X restores factor VIII hemostatic activity in a hemophilia A model. Nat Med. 2012;18:1570‐1574. - PubMed

