Effect of Once-Weekly Azithromycin vs Placebo in Children With HIV-Associated Chronic Lung Disease: The BREATHE Randomized Clinical Trial
- PMID: 33331916
- PMCID: PMC7747021
- DOI: 10.1001/jamanetworkopen.2020.28484
Effect of Once-Weekly Azithromycin vs Placebo in Children With HIV-Associated Chronic Lung Disease: The BREATHE Randomized Clinical Trial
Abstract
Importance: HIV-associated chronic lung disease (HCLD) in children is associated with small airways disease, is common despite antiretroviral therapy (ART), and is associated with substantial morbidity. Azithromycin has antibiotic and immunomodulatory activity and may be effective in treating HCLD through reducing respiratory tract infections and inflammation.
Objective: To determine whether prophylactic azithromycin is effective in preventing worsening of lung function and in reducing acute respiratory exacerbations (AREs) in children with HCLD taking ART.
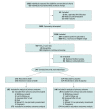
Design, setting, and participants: This double-blind, placebo-controlled, randomized clinical trial (BREATHE) was conducted between 2016 and 2019, including 12 months of follow-up, at outpatient HIV clinics in 2 public sector hospitals in Malawi and Zimbabwe. Participants were randomized 1:1 to intervention or placebo, and participants and study personnel were blinded to treatment allocation. Participants included children aged 6 to 19 years with perinatally acquired HIV and HCLD (defined as forced expiratory volume in 1 second [FEV1] z score < -1) who were taking ART for 6 months or longer. Data analysis was performed from September 2019 to April 2020.
Intervention: Once-weekly oral azithromycin with weight-based dosing, for 48 weeks.
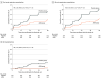
Main outcomes and measures: All outcomes were prespecified. The primary outcome was the mean difference in FEV1 z score using intention-to-treat analysis for participants seen at end line. Secondary outcomes included AREs, all-cause hospitalizations, mortality, and weight-for-age z score.
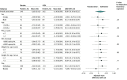
Results: A total of 347 individuals (median [interquartile range] age, 15.3 [12.7-17.7] years; 177 boys [51.0%]) were randomized, 174 to the azithromycin group and 173 to the placebo group; 162 participants in the azithromycin group and 146 placebo group participants had a primary outcome available and were analyzed. The mean difference in FEV1 z score was 0.06 (95% CI, -0.10 to 0.21; P = .48) higher in the azithromycin group than in the placebo group, a nonsignificant difference. The rate of AREs was 12.1 events per 100 person-years in the azithromycin group and 24.7 events per 100 person-years in the placebo groups (hazard ratio, 0.50; 95% CI, 0.27 to 0.93; P = .03). The hospitalization rate was 1.3 events per 100 person-years in the azithromycin group and 7.1 events per 100 person-years in the placebo groups, but the difference was not significant (hazard ratio, 0.24; 95% CI, 0.06 to 1.07; P = .06). Three deaths occurred, all in the placebo group. The mean weight-for-age z score was 0.03 (95% CI, -0.08 to 0.14; P = .56) higher in the azithromycin group than in the placebo group, although the difference was not significant. There were no drug-related severe adverse events.
Conclusions and relevance: In this randomized clinical trial specifically addressing childhood HCLD, once-weekly azithromycin did not improve lung function or growth but was associated with reduced AREs; the number of hospitalizations was also lower in the azithromycin group but the difference was not significant. Future research should identify patient groups who would benefit most from this intervention and optimum treatment length, to maximize benefits while reducing the risk of antimicrobial resistance.
Trial registration: ClinicalTrials.gov Identifier: NCT02426112.
Conflict of interest statement
Figures
References
-
- Joint United Nations Programme on HIV/AIDS UNAIDS data 2019. Published December 4, 2019. Accessed November 17, 2020. https://www.unaids.org/en/resources/documents/2019/2019-UNAIDS-data
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

