Assessment of Plasma Amyloid-β42/40 and Cognitive Decline Among Community-Dwelling Older Adults
- PMID: 33331917
- PMCID: PMC7747018
- DOI: 10.1001/jamanetworkopen.2020.28634
Assessment of Plasma Amyloid-β42/40 and Cognitive Decline Among Community-Dwelling Older Adults
Abstract
Importance: Plasma measurement of amyloid-β (Aβ) peptides has been associated with cognitive function, but evidence of its ability to identify cognitive decline is still scarce.
Objective: To investigate the associations between plasma Aβ42/40 and cognitive decline over time among community-dwelling older adults with subjective memory concerns.
Design, setting, and participants: This multicenter cohort study used data from volunteers in the 5-year study Multidomain Alzheimer Preventive Trial (MAPT). Participants were aged 70 years or older and observed for a median (interquartile range) of 3.9 (2.0-4.0) years. Recruitment of participants started in May 2008 and ended in February 2011. Follow-up ended in April 2016. Data analysis was conducted from April to October 2020.
Exposure: Plasma Aβ42 and Aβ40 were measured at 12 months for 448 participants (92.8%) and at 24 months for the rest. The moment of Aβ assessment was defined as the baseline for this study.
Main outcomes and measures: Cognitive function was assessed at 12, 24, 36, 48, and 60 months by a composite cognitive score based on 4 tests; Mini Mental State Examination (MMSE); Clinical Dementia Rating, sum of boxes; and Alzheimer Disease Cooperative Study-Activities of Daily Living. Mixed-effect linear regressions were performed.
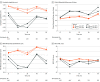
Results: A total of 483 participants (median [IQR] age, 76.0 [73.0-80.0]; 286 [59.2%] women) were analyzed. Of them, 161 (33.3%) were classified as low plasma Aβ42/40 (≤0.107). After adjusting for age, sex, education, body mass index, Geriatric Depression Scale score, apolipoprotein E ε4 genotype, and MAPT intervention groups, low plasma Aβ42/40 was associated with more pronounced decline in composite cognitive score (adjusted between-group mean difference: -0.20, 95% CI, -0.34 to -0.07; P = .004) and decline in MMSE score (adjusted between-group mean difference: -0.59; 95% CI, -1.07 to -0.11; P = .02) during the follow-up period compared with the group with an Aβ42/40 ratio greater than 0.107.
Conclusions and relevance: In this study, low plasma Aβ42/40 was associated with more pronounced decline in cognitive function (measured by multiple outcomes) over time. Findings suggest that plasma Aβ42/40 may be used to identify people at risk of cognitive decline, being an alternative to more complex and expensive measures, such as positron emission tomography imaging or cerebrospinal fluid measurement.
Conflict of interest statement
Figures
References
-
- Bateman RJ, Blennow K, Doody R, et al. Plasma biomarkers of AD emerging as essential tools for drug development: an EU/US CTAD Task Force report. J Prev Alzheimers Dis. 2019;6(3):169-173. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

