Gefitinib Versus Vinorelbine Plus Cisplatin as Adjuvant Treatment for Stage II-IIIA (N1-N2) EGFR-Mutant NSCLC: Final Overall Survival Analysis of CTONG1104 Phase III Trial
- PMID: 33332190
- PMCID: PMC8078324
- DOI: 10.1200/JCO.20.01820
Gefitinib Versus Vinorelbine Plus Cisplatin as Adjuvant Treatment for Stage II-IIIA (N1-N2) EGFR-Mutant NSCLC: Final Overall Survival Analysis of CTONG1104 Phase III Trial
Abstract
Purpose: ADJUVANT-CTONG1104 (ClinicalTrials.gov identifier: NCT01405079), a randomized phase III trial, showed that adjuvant gefitinib treatment significantly improved disease-free survival (DFS) versus vinorelbine plus cisplatin (VP) in patients with epidermal growth factor receptor (EGFR) mutation-positive resected stage II-IIIA (N1-N2) non-small-cell lung cancer (NSCLC). Here, we report the final overall survival (OS) results.
Methods: From September 2011 to April 2014, 222 patients from 27 sites were randomly assigned 1:1 to adjuvant gefitinib (n = 111) or VP (n = 111). Patients with resected stage II-IIIA (N1-N2) NSCLC and EGFR-activating mutation were enrolled, receiving gefitinib for 24 months or VP every 3 weeks for four cycles. The primary end point was DFS (intention-to-treat [ITT] population). Secondary end points included OS, 3-, 5-year (y) DFS rates, and 5-year OS rate. Post hoc analysis was conducted for subsequent therapy data.
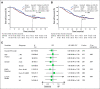
Results: Median follow-up was 80.0 months. Median OS (ITT) was 75.5 and 62.8 months with gefitinib and VP, respectively (hazard ratio [HR], 0.92; 95% CI, 0.62 to 1.36; P = .674); respective 5-year OS rates were 53.2% and 51.2% (P = .784). Subsequent therapy was administered upon progression in 68.4% and 73.6% of patients receiving gefitinib and VP, respectively. Subsequent targeted therapy contributed most to OS (HR, 0.23; 95% CI, 0.14 to 0.38) compared with no subsequent therapy. Updated 3y DFS rates were 39.6% and 32. 5% with gefitinib and VP (P = .316) and 5y DFS rates were 22. 6% and 23.2% (P = .928), respectively.
Conclusion: Adjuvant therapy with gefitinib in patients with early-stage NSCLC and EGFR mutation demonstrated improved DFS over standard of care chemotherapy. Although this DFS advantage did not translate to a significant OS difference, OS with adjuvant gefitinib was one of the longest observed in this patient group compared with historic data.
Figures



Comment in
-
Adjuvant Therapy With EGFR Tyrosine Kinase Inhibitors: Tempering Great Expectations With Realism.J Clin Oncol. 2021 Mar 1;39(7):697-700. doi: 10.1200/JCO.20.03297. Epub 2021 Jan 8. J Clin Oncol. 2021. PMID: 33417483 No abstract available.
References
-
- Cheng H Li XJ Wang XJ, et al. : A meta-analysis of adjuvant EGFR-TKIs for patients with resected non-small cell lung cancer. Lung Cancer 137:7-13, 2019 - PubMed
-
- Pisters KM Evans WK Azzoli CG, et al. : Cancer Care Ontario and American Society of Clinical Oncology adjuvant chemotherapy and adjuvant radiation therapy for stages I-IIIA resectable non small-cell lung cancer guideline. J Clin Oncol 25:5506-5518, 2007 - PubMed
-
- Xiu-Yi Zhi Jin-Ming Yu, and Yuan-Kai Shi. Chinese guidelines on the diagnosis and treatment of primary lung cancer (2015 version). Cancer 121:3165-3181, 2015. Cancer 122:162, 2016 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

