Update to CDC's Treatment Guidelines for Gonococcal Infection, 2020
- PMID: 33332296
- PMCID: PMC7745960
- DOI: 10.15585/mmwr.mm6950a6
Update to CDC's Treatment Guidelines for Gonococcal Infection, 2020
Abstract
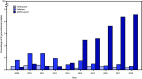
Sexually transmitted infections (STIs) caused by the bacteria Neisseria gonorrhoeae (gonococcal infections) have increased 63% since 2014 and are a cause of sequelae including pelvic inflammatory disease, ectopic pregnancy, and infertility and can facilitate transmission of human immunodeficiency virus (HIV) (1,2). Effective treatment can prevent complications and transmission, but N. gonorrhoeae's ability to acquire antimicrobial resistance influences treatment recommendations and complicates control (3). In 2010, CDC recommended a single 250 mg intramuscular (IM) dose of ceftriaxone and a single 1 g oral dose of azithromycin for treatment of uncomplicated gonococcal infections of the cervix, urethra, and rectum as a strategy for preventing ceftriaxone resistance and treating possible coinfection with Chlamydia trachomatis (4). Increasing concern for antimicrobial stewardship and the potential impact of dual therapy on commensal organisms and concurrent pathogens (3), in conjunction with the continued low incidence of ceftriaxone resistance and the increased incidence of azithromycin resistance, has led to reevaluation of this recommendation. This report, which updates previous guidelines (5), recommends a single 500 mg IM dose of ceftriaxone for treatment of uncomplicated urogenital, anorectal, and pharyngeal gonorrhea. If chlamydial infection has not been excluded, concurrent treatment with doxycycline (100 mg orally twice a day for 7 days) is recommended. Continuing to monitor for emergence of ceftriaxone resistance through surveillance and health care providers' reporting of treatment failures is essential to ensuring continued efficacy of recommended regimens.
Conflict of interest statement
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. Lindley Barbee reports a grant from SpeeDx and from Nabriva, personal fees from Nabriva, and nonfinancial support from Hologic, outside the submitted work. No other potential conflicts of interest were disclosed.
Figures
References
-
- CDC. Sexually transmitted disease surveillance 2018. Atlanta, GA: US Department of Health and Human Services, CDC; 2019. https://www.cdc.gov/std/stats18/STDSurveillance2018-full-report.pdf
-
- CDC. Antibiotic resistance threats in the United States, 2019. Atlanta, GA: US Department of Health and Human Services, CDC; 2019. https://www.cdc.gov/drugresistance/pdf/threats-report/2019-ar-threats-re...
-
- Workowski KA, Berman S; CDC. Sexually transmitted diseases treatment guidelines, 2010. MMWR Recomm Rep 2010;59(No. RR-12). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

