Pre-test probability for SARS-Cov-2-related infection score: The PARIS score
- PMID: 33332360
- PMCID: PMC7745977
- DOI: 10.1371/journal.pone.0243342
Pre-test probability for SARS-Cov-2-related infection score: The PARIS score
Abstract
Introduction: In numerous countries, large population testing is impossible due to the limited availability of RT-PCR kits and CT-scans. This study aimed to determine a pre-test probability score for SARS-CoV-2 infection.
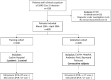
Methods: This multicenter retrospective study (4 University Hospitals) included patients with clinical suspicion of SARS-CoV-2 infection. Demographic characteristics, clinical symptoms, and results of blood tests (complete white blood cell count, serum electrolytes and CRP) were collected. A pre-test probability score was derived from univariate analyses of clinical and biological variables between patients and controls, followed by multivariate binary logistic analysis to determine the independent variables associated with SARS-CoV-2 infection.
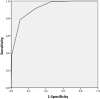
Results: 605 patients were included between March 10th and April 30th, 2020 (200 patients for the training cohort, 405 consecutive patients for the validation cohort). In the multivariate analysis, lymphocyte (<1.3 G/L), eosinophil (<0.06 G/L), basophil (<0.04 G/L) and neutrophil counts (<5 G/L) were associated with high probability of SARS-CoV-2 infection but no clinical variable was statistically significant. The score had a good performance in the validation cohort (AUC = 0.918 (CI: [0.891-0.946]; STD = 0.014) with a Positive Predictive Value of high-probability score of 93% (95%CI: [0.89-0.96]). Furthermore, a low-probability score excluded SARS-CoV-2 infection with a Negative Predictive Value of 98% (95%CI: [0.93-0.99]). The performance of the score was stable even during the last period of the study (15-30th April) with more controls than infected patients.
Conclusions: The PARIS score has a good performance to categorize the pre-test probability of SARS-CoV-2 infection based on complete white blood cell count. It could help clinicians adapt testing and for rapid triage of patients before test results.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis [Internet]. 2020. February 19 [cited 2020 May 5]; Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7159018/ - PMC - PubMed
-
- Onder G, Rezza G, Brusaferro S. Case-Fatality Rate and Characteristics of Patients Dying in Relation to COVID-19 in Italy. JAMA [Internet]. 2020. March 23 [cited 2020 May 5]; Available from: https://jamanetwork.com/journals/jama/fullarticle/2763667 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous