Tocilizumab in Patients Hospitalized with Covid-19 Pneumonia
- PMID: 33332779
- PMCID: PMC7781101
- DOI: 10.1056/NEJMoa2030340
Tocilizumab in Patients Hospitalized with Covid-19 Pneumonia
Abstract
Background: Coronavirus disease 2019 (Covid-19) pneumonia is often associated with hyperinflammation. Despite the disproportionate incidence of Covid-19 among underserved and racial and ethnic minority populations, the safety and efficacy of the anti-interleukin-6 receptor antibody tocilizumab in patients from these populations who are hospitalized with Covid-19 pneumonia are unclear.
Methods: We randomly assigned (in a 2:1 ratio) patients hospitalized with Covid-19 pneumonia who were not receiving mechanical ventilation to receive standard care plus one or two doses of either tocilizumab (8 mg per kilogram of body weight intravenously) or placebo. Site selection was focused on the inclusion of sites enrolling high-risk and minority populations. The primary outcome was mechanical ventilation or death by day 28.
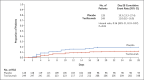
Results: A total of 389 patients underwent randomization, and the modified intention-to-treat population included 249 patients in the tocilizumab group and 128 patients in the placebo group; 56.0% were Hispanic or Latino, 14.9% were Black, 12.7% were American Indian or Alaska Native, 12.7% were non-Hispanic White, and 3.7% were of other or unknown race or ethnic group. The cumulative percentage of patients who had received mechanical ventilation or who had died by day 28 was 12.0% (95% confidence interval [CI], 8.5 to 16.9) in the tocilizumab group and 19.3% (95% CI, 13.3 to 27.4) in the placebo group (hazard ratio for mechanical ventilation or death, 0.56; 95% CI, 0.33 to 0.97; P = 0.04 by the log-rank test). Clinical failure as assessed in a time-to-event analysis favored tocilizumab over placebo (hazard ratio, 0.55; 95% CI, 0.33 to 0.93). Death from any cause by day 28 occurred in 10.4% of the patients in the tocilizumab group and 8.6% of those in the placebo group (weighted difference, 2.0 percentage points; 95% CI, -5.2 to 7.8). In the safety population, serious adverse events occurred in 38 of 250 patients (15.2%) in the tocilizumab group and 25 of 127 patients (19.7%) in the placebo group.
Conclusions: In hospitalized patients with Covid-19 pneumonia who were not receiving mechanical ventilation, tocilizumab reduced the likelihood of progression to the composite outcome of mechanical ventilation or death, but it did not improve survival. No new safety signals were identified. (Funded by Genentech; EMPACTA ClinicalTrials.gov number, NCT04372186.).
Copyright © 2020 Massachusetts Medical Society.
Figures
Comment in
-
Tocilizumab in Patients Hospitalized with Covid-19 Pneumonia.N Engl J Med. 2021 Apr 15;384(15):1473. doi: 10.1056/NEJMc2100217. Epub 2021 Mar 3. N Engl J Med. 2021. PMID: 33657286 No abstract available.
References
-
- Phelan AL, Katz R, Gostin LO. The novel coronavirus originating in Wuhan, China: challenges for global health governance. JAMA 2020;323:709-710. - PubMed
-
- World Health Organization. COVID-19 Public Health Emergency of International Concern (PHEIC) global research and innovation forum. 2020. (https://www.who.int/publications/m/item/covid-19-public-health-emergency...).
-
- Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA 2020;323:1239-1242. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
