The pyruvate-lactate axis modulates cardiac hypertrophy and heart failure
- PMID: 33333007
- PMCID: PMC7933116
- DOI: 10.1016/j.cmet.2020.12.003
The pyruvate-lactate axis modulates cardiac hypertrophy and heart failure
Abstract
The metabolic rewiring of cardiomyocytes is a widely accepted hallmark of heart failure (HF). These metabolic changes include a decrease in mitochondrial pyruvate oxidation and an increased export of lactate. We identify the mitochondrial pyruvate carrier (MPC) and the cellular lactate exporter monocarboxylate transporter 4 (MCT4) as pivotal nodes in this metabolic axis. We observed that cardiac assist device-induced myocardial recovery in chronic HF patients was coincident with increased myocardial expression of the MPC. Moreover, the genetic ablation of the MPC in cultured cardiomyocytes and in adult murine hearts was sufficient to induce hypertrophy and HF. Conversely, MPC overexpression attenuated drug-induced hypertrophy in a cell-autonomous manner. We also introduced a novel, highly potent MCT4 inhibitor that mitigated hypertrophy in cultured cardiomyocytes and in mice. Together, we find that alteration of the pyruvate-lactate axis is a fundamental and early feature of cardiac hypertrophy and failure.
Keywords: LVAD; MCT4; MPC; VB124; cardiac metabolism; heart failure; hypertrophy; lactate; mitochondria; pyruvate.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The University of Utah has filed a patent related to the mitochondrial pyruvate carrier, of which J.R. is listed as co-inventor. J.R. is a founder of Vettore Biosciences and a member of its scientific advisory board. K.M.P. is an employee and shareholder of Vettore Biosciences. S.M. was an employee of Vettore Biosciences. S.G.D. is a consultant to Abbott (Steering Committee member of the INTELLECT-2 multicenter trial of LVAD and CardioMEMS). J.R. and S.G.D. are the recipients of a grant from Merck related to mechanisms of HF and myocardial recovery. All other authors declare no competing interests.
Figures


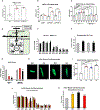

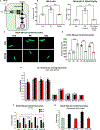


References
-
- ACIN-PEREZ R, LECHUGA-VIECO AV, DEL MAR MUNOZ M, NIETO-ARELLANO R, TORROJA C, SANCHEZ-CABO F, JIMENEZ C, GONZALEZ-GUERRA A, CARRASCOSO I, BENINCA C, QUIROS PM, LOPEZ-OTIN C, CASTELLANO JM, RUIZ-CABELLO J, JIMENEZ-BORREGUERO LJ & ENRIQUEZ JA 2018. Ablation of the stress protease OMA1 protects against heart failure in mice. Sci Transl Med, 10. - PubMed
-
- ALLARD MF, SCHONEKESS BO, HENNING SL, ENGLISH DR & LOPASCHUK GD 1994. Contribution of oxidative metabolism and glycolysis to ATP production in hypertrophied hearts. Am J Physiol, 267, H742–50. - PubMed
-
- BADOLIA R, RAMADURAI DKA, ABEL ED, FERRIN P, TALEB I, SHANKAR TS, KROKIDI AT, NAVANKASATTUSAS S, MCKELLAR SH, YIN M, KFOURY AG, WEVER-PINZON O, FANG JC, SELZMAN CH, CHAUDHURI D, RUTTER J & DRAKOS SG 2020. The Role of Nonglycolytic Glucose Metabolism in Myocardial Recovery Upon Mechanical Unloading and Circulatory Support in Chronic Heart Failure. Circulation, 142, 259–274. - PMC - PubMed
-
- BARGER PM & KELLY DP 1999. Fatty acid utilization in the hypertrophied and failing heart: molecular regulatory mechanisms. Am J Med Sci, 318, 36–42. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK108833/DK/NIDDK NIH HHS/United States
- R35 GM131854/GM/NIGMS NIH HHS/United States
- R01 HL135121/HL/NHLBI NIH HHS/United States
- U54 DK110858/DK/NIDDK NIH HHS/United States
- R01 HL132067/HL/NHLBI NIH HHS/United States
- K00 CA212445/CA/NCI NIH HHS/United States
- T32 DK091317/DK/NIDDK NIH HHS/United States
- R01 DK112826/DK/NIDDK NIH HHS/United States
- T32 HL007576/HL/NHLBI NIH HHS/United States
- T32 DK110966/DK/NIDDK NIH HHS/United States
- S10 OD016232/OD/NIH HHS/United States
- S10 OD018210/OD/NIH HHS/United States
- R00 CA215307/CA/NCI NIH HHS/United States
- S10 OD021505/OD/NIH HHS/United States
- R01 CA228346/CA/NCI NIH HHS/United States
- F99 CA253744/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

