Defining and managing COVID-19-associated pulmonary aspergillosis: the 2020 ECMM/ISHAM consensus criteria for research and clinical guidance
- PMID: 33333012
- PMCID: PMC7833078
- DOI: 10.1016/S1473-3099(20)30847-1
Defining and managing COVID-19-associated pulmonary aspergillosis: the 2020 ECMM/ISHAM consensus criteria for research and clinical guidance
Abstract
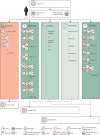
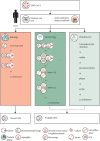
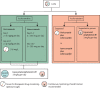
Severe acute respiratory syndrome coronavirus 2 causes direct damage to the airway epithelium, enabling aspergillus invasion. Reports of COVID-19-associated pulmonary aspergillosis have raised concerns about it worsening the disease course of COVID-19 and increasing mortality. Additionally, the first cases of COVID-19-associated pulmonary aspergillosis caused by azole-resistant aspergillus have been reported. This article constitutes a consensus statement on defining and managing COVID-19-associated pulmonary aspergillosis, prepared by experts and endorsed by medical mycology societies. COVID-19-associated pulmonary aspergillosis is proposed to be defined as possible, probable, or proven on the basis of sample validity and thus diagnostic certainty. Recommended first-line therapy is either voriconazole or isavuconazole. If azole resistance is a concern, then liposomal amphotericin B is the drug of choice. Our aim is to provide definitions for clinical research and up-to-date recommendations for clinical management of the diagnosis and treatment of COVID-19-associated pulmonary aspergillosis.
Copyright © 2021 Elsevier Ltd. All rights reserved.
Figures
Comment in
-
Diagnostic dilemma in COVID-19-associated pulmonary aspergillosis - Authors' reply.Lancet Infect Dis. 2021 Jun;21(6):767-769. doi: 10.1016/S1473-3099(21)00123-7. Epub 2021 Mar 1. Lancet Infect Dis. 2021. PMID: 33662323 Free PMC article. No abstract available.
-
Diagnostic dilemma in COVID-19-associated pulmonary aspergillosis.Lancet Infect Dis. 2021 Jun;21(6):766-767. doi: 10.1016/S1473-3099(21)00060-8. Epub 2021 Mar 1. Lancet Infect Dis. 2021. PMID: 33662327 Free PMC article. No abstract available.
References
-
- Schauwvlieghe AFAD, Rijnders BJA, Philips N, et al. Invasive aspergillosis in patients admitted to the intensive care unit with severe influenza: a retrospective cohort study. Lancet Respir Med. 2018;6:782–792. - PubMed
-
- Vehreschild JJ, Bröckelmann PJ, Bangard C, et al. Pandemic 2009 influenza A(H1N1) virus infection coinciding with invasive pulmonary aspergillosis in neutropenic patients. Epidemiol Infect. 2012;140:18–52. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

