Effectiveness of adjunctive clindamycin in β-lactam antibiotic-treated patients with invasive β-haemolytic streptococcal infections in US hospitals: a retrospective multicentre cohort study
- PMID: 33333013
- PMCID: PMC8084921
- DOI: 10.1016/S1473-3099(20)30523-5
Effectiveness of adjunctive clindamycin in β-lactam antibiotic-treated patients with invasive β-haemolytic streptococcal infections in US hospitals: a retrospective multicentre cohort study
Erratum in
-
Correction to Lancet Infect Dis 2021; 21: 697-710.Lancet Infect Dis. 2021 May;21(5):e122. doi: 10.1016/S1473-3099(21)00228-0. Lancet Infect Dis. 2021. PMID: 33894852 No abstract available.
Abstract
Background: Clindamycin is strongly recommended as an adjunctive treatment to β-lactam antibiotics in patients with severe invasive group A β-haemolytic streptococcal (iGAS) infections. However, there is little evidence of a benefit in the use of clindamycin in humans, and its role, if any, in treating patients with invasive non-group A/B β-haemolytic streptococcal (iNABS) infections is unclear.
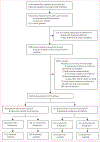
Methods: For this retrospective multicentre cohort study, we used a dataset from patients in the Cerner Health Facts database, which contains electronic health-based data from 233 US hospitals. We queried the Cerner Health Facts database for inpatients (no age restriction) admitted to hospital in 2000-15, with any clinical cultures positive for β-haemolytic streptococcal taxa of interest, and who had received β-lactam antibiotics within 3 days either side of culture sampling. This group of patients was then queried for those who had also received intravenous or oral clindamycin within 3 days either side of culture sampling. Patients were excluded if they had polymicrobial growth or clindamycin non-susceptible isolates, received linezolid, or had missing variable data needed for analysis. Patients were categorised by Lancefield group (iGAS or iNABS); β-lactam antibiotic-treated patients who had received clindamycin were propensity-matched (1:2) to those who did not receive clindamycin separately for iGAS and iNABS cohorts, and logistic regression was then used to account for residual confounding factors. The primary outcome was the adjusted odds ratio (aOR) of in-hospital mortality in propensity-matched patients treated with adjunctive clindamycin versus those not treated with clindamycin in the iGAS and iNABS infection cohorts.
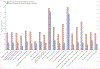
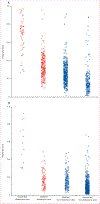
Findings: We identified 1956 inpatients with invasive β-haemolytic streptococcal infection who had been treated with β-lactam antibiotics across 118 hospitals (1079 with iGAS infections and 877 with iNABS infections). 459 (23·4%) of these patients had received adjunctive clindamycin treatment (343 [31·7%] patients with iGAS infections and 116 [13·2%] patients with iNABS infections). The effect of adjunctive clindamycin therapy on in-hospital mortality differed significantly and showed the opposite trend in iGAS and iNABS infection cohorts (p=0·013 for an interaction). In the iGAS cohort, in-hospital mortality in propensity-matched patients who received adjunctive clindamycin (18 [6·5%] of 277 patients) was significantly lower than in those who did not (55 [11·0%] of 500 patients; aOR 0·44 [95% CI 0·23-0·81]). This survival benefit was maintained even in patients without shock or necrotising fasciitis (six [2·6%] of 239 patients treated with adjunctive clindamycin vs 27 [6·1%] of 422 patients not treated with adjunctive clindamycin; aOR 0·40 [0·15-0·91]). By contrast, in the iNABS infection cohort, in-hospital mortality in propensity-matched patients who received adjunctive clindamycin (ten [9·8%] of 102) was higher than in those who did not (nine [4·6%] of 193), but this difference was not significant (aOR 2·60 [0·94-7·52]). Several subset analyses found qualitatively similar results.
Interpretation: Real-world data suggest that increased use of adjunctive clindamycin for invasive iGAS infections, but not iNABS infections, could improve outcomes, even in patients without shock or necrotising fasciitis.
Funding: Intramural Research Program of the National Institutes of Health Clinical Center and the National Institute of Allergy and Infectious Disease.
Copyright © 2021 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests
We declare no competing interests.
Figures
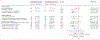
Comment in
-
Adjunctive clindamycin therapy in invasive β-haemolytic streptococcal infections - Authors' reply.Lancet Infect Dis. 2021 Jun;21(6):762-763. doi: 10.1016/S1473-3099(21)00259-0. Lancet Infect Dis. 2021. PMID: 34051182 No abstract available.
-
Adjunctive clindamycin therapy in invasive β-haemolytic streptococcal infections.Lancet Infect Dis. 2021 Jun;21(6):762. doi: 10.1016/S1473-3099(21)00198-5. Lancet Infect Dis. 2021. PMID: 34051183 No abstract available.
-
Increasing clindamycin resistance in group A streptococcus.Lancet Infect Dis. 2021 Sep;21(9):1208-1209. doi: 10.1016/S1473-3099(21)00456-4. Lancet Infect Dis. 2021. PMID: 34450068 No abstract available.
References
-
- Carapetis JR, Steer AC, Mulholland EK, Weber M. The global burden of group A streptococcal diseases. Lancet Infect Dis 2005; 5: 685–94. - PubMed
-
- O’Loughlin RE, Roberson A, Cieslalc PR, et al. The epidemiology of invasive group A streptococcal infection and potential vaccine implications: United States, 2000–2004. Clin Infect Dis 2007; 45: 853–62. - PubMed
-
- Srislcandan S, Ferguson M, Elliot V, Faulkner L, Cohen J. Human intravenous immunoglobulin for experimental streptococcal toxic shock: bacterial clearance and modulation of inflammation. J Antimicrob Chemother 2006; 58: 117–24. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

