Experimental design approach for development of spectrofluorimetric method for determination of favipiravir; a potential therapeutic agent against COVID-19 virus: Application to spiked human plasma
- PMID: 33333412
- PMCID: PMC7834856
- DOI: 10.1016/j.saa.2020.119241
Experimental design approach for development of spectrofluorimetric method for determination of favipiravir; a potential therapeutic agent against COVID-19 virus: Application to spiked human plasma
Abstract
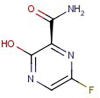
The present work describes development of rapid, robust, sensitive and green spectrofluorimetric method for determination of favipiravir (FAV). Different factors affecting fluorescence were carefully studied and Box Behnken Design was applied to optimize experimental parameters. The proposed method is based on measuring native fluorescence of FAV in 0.2 M borate buffer (pH 8.0) at 432 nm after excitation at 361 nm. There was a linear relationship between FAV concentration and relative fluorescence intensity over the range 40-280 ng/mL with limit of detection of 9.44 ng/mL and quantitation limit of 28.60 ng/mL. The method was successfully implemented for determination of FAV in its pharmaceutical formulation with mean % recovery of 99.26 ± 0.87. Moreover, the high sensitivity of the method allowed determination of FAV in spiked human plasma over a range of 48-192 ng/mL. The proposed spectrofluorimetric method was proved to be eco-friendly according to analytical eco-scale.
Keywords: COVID-19 virus; Experimental design; Favipiravir; Human plasma; Pharmaceutical analysis.
Copyright © 2020 Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




Similar articles
-
An ultrasensitive green synchronous factorized spectrofluorimetric approach for the simultaneous determination of Favipiravir and Molnupiravir: Application to pharmaceutical, environmental, and biological matrices.Luminescence. 2024 Aug;39(8):e4837. doi: 10.1002/bio.4837. Luminescence. 2024. PMID: 39113185
-
Development and validation of a sensitive, fast and simple LC-MS / MS method for the quantitation of favipiravir in human serum.J Chromatogr B Analyt Technol Biomed Life Sci. 2021 Jun 30;1176:122768. doi: 10.1016/j.jchromb.2021.122768. Epub 2021 May 20. J Chromatogr B Analyt Technol Biomed Life Sci. 2021. PMID: 34052564 Free PMC article.
-
Development and Validation of a Method for Quantification of Favipiravir as COVID-19 Management in Spiked Human Plasma.Molecules. 2021 Jun 22;26(13):3789. doi: 10.3390/molecules26133789. Molecules. 2021. PMID: 34206357 Free PMC article.
-
Favipiravir use for SARS CoV-2 infection.Pharmacol Rep. 2020 Dec;72(6):1542-1552. doi: 10.1007/s43440-020-00175-2. Epub 2020 Oct 27. Pharmacol Rep. 2020. PMID: 33108587 Free PMC article. Review.
-
Favipiravir and COVID-19: A Simplified Summary.Drug Res (Stuttg). 2021 Mar;71(3):166-170. doi: 10.1055/a-1296-7935. Epub 2020 Nov 11. Drug Res (Stuttg). 2021. PMID: 33176367 Free PMC article. Review.
Cited by
-
Structural Elucidation of Alkali Degradation Impurities of Favipiravir from the Oral Suspension: UPLC-TQ-ESI-MS/MS and NMR.Molecules. 2022 Aug 31;27(17):5606. doi: 10.3390/molecules27175606. Molecules. 2022. PMID: 36080375 Free PMC article.
-
An unusual case of bluish discoloration of the cornea after favipiravir therapy for COVID-19.Indian J Ophthalmol. 2021 Dec;69(12):3778-3779. doi: 10.4103/ijo.IJO_1023_21. Indian J Ophthalmol. 2021. PMID: 34827050 Free PMC article. No abstract available.
-
Synergistic effect of gold nanoparticles anchored on conductive carbon black as an efficient electrochemical sensor for sensitive detection of anti-COVID-19 drug Favipiravir in absence and presence of co-administered drug Paracetamol.Microchem J. 2023 Jul;190:108696. doi: 10.1016/j.microc.2023.108696. Epub 2023 Mar 31. Microchem J. 2023. PMID: 37034437 Free PMC article.
-
Eco-sustainable chromatographic method for the determination of favipiravir and nitazoxanide for COVID-19: application to human plasma.BMC Chem. 2025 Jan 9;19(1):11. doi: 10.1186/s13065-024-01364-3. BMC Chem. 2025. PMID: 39789629 Free PMC article.
-
Green micellar solvent-free HPLC and spectrofluorimetric determination of favipiravir as one of COVID-19 antiviral regimens.Microchem J. 2021 Jun;165:106189. doi: 10.1016/j.microc.2021.106189. Epub 2021 Mar 23. Microchem J. 2021. PMID: 33776146 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

