Current Status on Therapeutic Molecules Targeting Siglec Receptors
- PMID: 33333862
- PMCID: PMC7765293
- DOI: 10.3390/cells9122691
Current Status on Therapeutic Molecules Targeting Siglec Receptors
Abstract
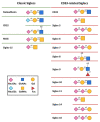
The sialic acid-binding immunoglobulin-type of lectins (Siglecs) are receptors that recognize sialic acid-containing glycans. In the majority of the cases, Siglecs are expressed on immune cells and play a critical role in regulating immune cell signaling. Over the years, it has been shown that the sialic acid-Siglec axis participates in immunological homeostasis, and that any imbalance can trigger different pathologies, such as autoimmune diseases or cancer. For all this, different therapeutics have been developed that bind to Siglecs, either based on antibodies or being smaller molecules. In this review, we briefly introduce the Siglec family and we compile a description of glycan-based molecules and antibody-based therapies (including CAR-T and bispecific antibodies) that have been designed to therapeutically targeting Siglecs.
Keywords: CAR; Siglec; antibody; glycan; sialic acid.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Diaz S.L., Padler-Karavani V., Ghaderi D., Hurtado-Ziola N., Yu H., Chen X., Brinkman-Van der Linden E.C.M., Varki A., Varki N.M. Sensitive and specific detection of the non-human sialic acid N-Glycolylneuraminic acid in human tissues and biotherapeutic products. PLoS ONE. 2009;4:e4241. doi: 10.1371/journal.pone.0004241. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

