Cyclodextrin-Based Functional Glyconanomaterials
- PMID: 33333914
- PMCID: PMC7765426
- DOI: 10.3390/nano10122517
Cyclodextrin-Based Functional Glyconanomaterials
Abstract
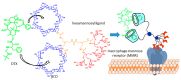
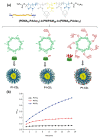
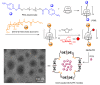
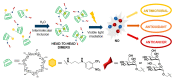
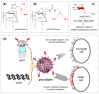
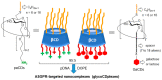
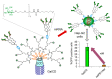
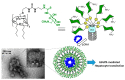
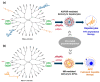
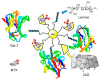
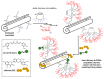
Cyclodextrins (CDs) have long occupied a prominent position in most pharmaceutical laboratories as "off-the-shelve" tools to manipulate the pharmacokinetics of a broad range of active principles, due to their unique combination of biocompatibility and inclusion abilities. The development of precision chemical methods for their selective functionalization, in combination with "click" multiconjugation procedures, have further leveraged the nanoscaffold nature of these oligosaccharides, creating a direct link between the glyco and the nano worlds. CDs have greatly contributed to understand and exploit the interactions between multivalent glycodisplays and carbohydrate-binding proteins (lectins) and to improve the drug-loading and functional properties of nanomaterials through host-guest strategies. The whole range of capabilities can be enabled through self-assembly, template-assisted assembly or covalent connection of CD/glycan building blocks. This review discusses the advancements made in this field during the last decade and the amazing variety of functional glyconanomaterials empowered by the versatility of the CD component.
Keywords: cyclodextrins; drug delivery; gene delivery; glyconanomaterials; glyconanoparticles; glycopolymers; glycotargeting; multivalency; self-assembly; supramolecular nanomaterials.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








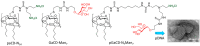



References
-
- Tian B., Xiao D., Hei T., Ping R., Hua S., Liu J. The application and prospects of cyclodextrin inclusion complexes and polymers in the food industry: A review. Polym. Int. 2020;69:597–603. doi: 10.1002/pi.5992. - DOI
-
- Matencio A., Navarro-Orcajada S., García-Carmona F., López-Nicolás J.M. Applications of cyclodextrins in food science. A review. Trends Food Sci. Technol. 2020;104:132–143. doi: 10.1016/j.tifs.2020.08.009. - DOI
-
- Ortiz Mellet C., García Fernández J.M., Benito J.M. Cyclodextrins for pharmacological and biomedical applications. In: Schneider H.-J., editor. Supramolecular Systems in Biomedical Fields. Royal Society of Chemistry; Cambridge, UK: 2013. pp. 94–139. Chapter 5.
-
- Fakayode S.O., Lowry M., Fletcher K.A., Huang X., Powe A.M., Warner I.M. Cyclodextrins host-guest chemistry in analytical and environmental chemistry. Curr. Anal. Chem. 2007;3:171–181. doi: 10.2174/157341107781023811. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

