Block-And-Lock: New Horizons for a Cure for HIV-1
- PMID: 33334019
- PMCID: PMC7765451
- DOI: 10.3390/v12121443
Block-And-Lock: New Horizons for a Cure for HIV-1
Abstract
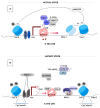
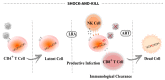
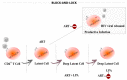
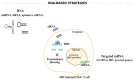
HIV-1/AIDS remains a global public health problem. The world health organization (WHO) reported at the end of 2019 that 38 million people were living with HIV-1 worldwide, of which only 67% were accessing antiretroviral therapy (ART). Despite great success in the clinical management of HIV-1 infection, ART does not eliminate the virus from the host genome. Instead, HIV-1 remains latent as a viral reservoir in any tissue containing resting memory CD4+ T cells. The elimination of these residual proviruses that can reseed full-blown infection upon treatment interruption remains the major barrier towards curing HIV-1. Novel approaches have recently been developed to excise or disrupt the virus from the host cells (e.g., gene editing with the CRISPR-Cas system) to permanently shut off transcription of the virus (block-and-lock and RNA interference strategies), or to reactivate the virus from cell reservoirs so that it can be eliminated by the immune system or cytopathic effects (shock-and-kill strategy). Here, we will review each of these approaches, with the major focus placed on the block-and-lock strategy.
Keywords: HIV-1 latency; HIV-1 reservoir; antiretroviral therapy; block-and-lock; functional cure.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- World Health Organization (WHO) HIV-AIDS. WHO; Geneva, Switzerland: 2020. Fact sheets.
-
- Hamlyn E., Ewings F.M., Porter K., Cooper D.A., Tambussi G., Schechter M., Pedersen C., Okulicz J.F., McClure M., Babiker A., et al. Plasma HIV Viral Rebound following Protocol-Indicated Cessation of ART Commenced in Primary and Chronic HIV Infection. PLoS ONE. 2012;7:e43754. doi: 10.1371/journal.pone.0043754. - DOI - PMC - PubMed
-
- Bahrami H., Budoff M., Haberlen S.A., Rezaeian P., Ketlogetswe K., Tracy R., Palella F., Witt M.D., McConnell M.V., Kingsley L., et al. Inflammatory markers associated with subclinical coronary artery disease: The multicenter AIDS cohort study. J. Am. Heart Assoc. 2016;5:e003371. doi: 10.1161/JAHA.116.003371. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

