TGF-β Serum Levels in Diabetic Retinopathy Patients and the Role of Anti-VEGF Therapy
- PMID: 33334029
- PMCID: PMC7765505
- DOI: 10.3390/ijms21249558
TGF-β Serum Levels in Diabetic Retinopathy Patients and the Role of Anti-VEGF Therapy
Abstract
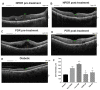
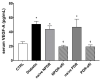
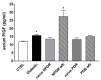
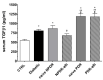
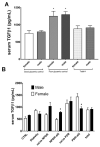
Transforming growth factor β1 (TGFβ1) is a proinflammatory cytokine that has been implicated in the pathogenesis of diabetic retinopathy (DR), particularly in the late phase of disease. The aim of the present study was to validate serum TGFβ1 as a diagnostic and prognostic biomarker of DR stages. Thirty-eight subjects were enrolled and, after diagnosis and evaluation of inclusion and exclusion criteria, were assigned to six groups: (1) healthy age-matched control, (2) diabetic without DR, (3) non-proliferative diabetic retinopathy (NPDR) naïve to treatment, (4) NPDR treated with intravitreal (IVT) aflibercept, (5) proliferative diabetic retinopathy (PDR) naïve to treatment and (6) PDR treated with IVT aflibercept. Serum levels of vascular endothelial growth factor A (VEGF-A), placental growth factor (PlGF) and TGFβ1 were measured by means of enzyme-linked immunosorbent assay (ELISA). Foveal macular thickness (FMT) in enrolled subjects was evaluated by means of structural-optical coherence tomography (S-OCT). VEGF-A serum levels decreased in NPDR and PDR patients treated with aflibercept, compared to naïve DR patients. PlGF serum levels were modulated only in aflibercept-treated NPDR patients. Particularly, TGFβ1 serum levels were predictive of disease progression from NPDR to PDR. A Multivariate ANOVA analysis (M-ANOVA) was also carried out to assess the effects of fixed factors on glycated hemoglobin (HbA1c) levels, TGFβ1, and diabetes duration. In conclusion, our data have strengthened the hypothesis that TGFβ1 would be a biomarker and pharmacological target of diabetic retinopathy.
Keywords: TGFβ; anti-VEGFA; diabetic retinopathy; serum biomarkers.
Conflict of interest statement
Authors have no conflict of interest to declare.
Figures

References
-
- Parravano M., De Geronimo D., Scarinci F., Querques L., Virgili G., Simonett J.M., Varano M., Bandello F., Querques G. Diabetic Microaneurysms Internal Reflectivity on Spectral-Domain Optical Coherence Tomography and Optical Coherence Tomography Angiography Detection. Am. J. Ophthalmol. 2017;179:90–96. doi: 10.1016/j.ajo.2017.04.021. - DOI - PubMed
-
- Lee C.S., Lee A.Y., Baughman D., Sim D., Akelere T., Brand C., Crabb D.P., Denniston A.K., Downey L., Fitt A., et al. The United Kingdom Diabetic Retinopathy Electronic Medical Record Users Group: Report 3: Baseline Retinopathy and Clinical Features Predict Progression of Diabetic Retinopathy. Am. J. Ophthalmol. 2017;180:64–71. doi: 10.1016/j.ajo.2017.05.020. - DOI - PMC - PubMed

