Plasmodial Kinase Inhibitors Targeting Malaria: Recent Developments
- PMID: 33334080
- PMCID: PMC7765515
- DOI: 10.3390/molecules25245949
Plasmodial Kinase Inhibitors Targeting Malaria: Recent Developments
Abstract
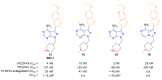
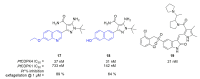
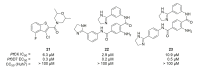
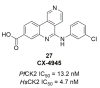
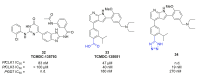
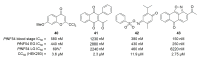
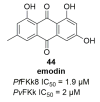
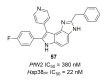
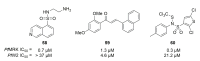
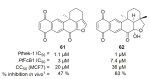
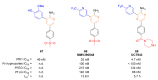
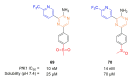
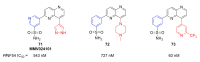
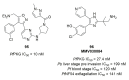
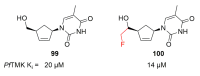
Recent progress in reducing malaria cases and ensuing deaths is threatened by factors like mutations that induce resistance to artemisinin derivatives. Multiple drugs are currently in clinical trials for malaria treatment, including some with novel mechanisms of action. One of these, MMV390048, is a plasmodial kinase inhibitor. This review lists the recently developed molecules which target plasmodial kinases. A systematic review of the literature was performed using CAPLUS and MEDLINE databases from 2005 to 2020. It covers a total of 60 articles and describes about one hundred compounds targeting 22 plasmodial kinases. This work highlights the strong potential of compounds targeting plasmodial kinases for future drug therapies. However, the majority of the Plasmodium kinome remains to be explored.
Keywords: Plasmodium; SAR; kinase inhibitor; malaria; medicinal chemistry.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
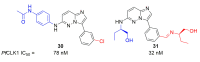
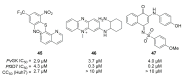
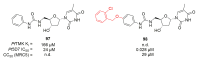
Similar articles
-
Targeting malaria protein kinases.Adv Protein Chem Struct Biol. 2021;124:225-274. doi: 10.1016/bs.apcsb.2020.10.004. Epub 2020 Nov 1. Adv Protein Chem Struct Biol. 2021. PMID: 33632466 Review.
-
An Update on Development of Small-Molecule Plasmodial Kinase Inhibitors.Molecules. 2020 Nov 7;25(21):5182. doi: 10.3390/molecules25215182. Molecules. 2020. PMID: 33171706 Free PMC article. Review.
-
Targeting the vitamin biosynthesis pathways for the treatment of malaria.Future Med Chem. 2013 May;5(7):769-79. doi: 10.4155/fmc.13.43. Future Med Chem. 2013. PMID: 23651091 Review.
-
Plasmodial Kinase Inhibitors: License to Cure?J Med Chem. 2018 Sep 27;61(18):8061-8077. doi: 10.1021/acs.jmedchem.8b00329. Epub 2018 Jun 4. J Med Chem. 2018. PMID: 29771541 Free PMC article.
-
Substituted imidazopyridazines are potent and selective inhibitors of Plasmodium falciparum calcium-dependent protein kinase 1 (PfCDPK1).Bioorg Med Chem Lett. 2013 May 15;23(10):3064-9. doi: 10.1016/j.bmcl.2013.03.017. Epub 2013 Mar 21. Bioorg Med Chem Lett. 2013. PMID: 23570789 Free PMC article.
Cited by
-
Dual RNA Sequencing Meta-analysis in Plasmodium Infection Identifies Host-Parasite Interactions.mSystems. 2021 Apr 20;6(2):e00182-21. doi: 10.1128/mSystems.00182-21. mSystems. 2021. PMID: 33879496 Free PMC article.
-
Targeting the Plasmodium falciparum proteome and organelles for potential antimalarial drug candidates.Heliyon. 2022 Aug;8(8):e10390. doi: 10.1016/j.heliyon.2022.e10390. Epub 2022 Aug 24. Heliyon. 2022. PMID: 36033316 Free PMC article. Review.
-
Efficacy, Safety, Tolerability, and Pharmacokinetics of MMV390048 in Acute Uncomplicated Malaria.Am J Trop Med Hyg. 2022 Dec 5;108(1):81-84. doi: 10.4269/ajtmh.22-0567. Print 2023 Jan 11. Am J Trop Med Hyg. 2022. PMID: 36509063 Free PMC article. Clinical Trial.
-
Employing Hexahydroquinolines as PfCDPK4 Inhibitors to Combat Malaria Transmission: An Advanced Computational Approach.Adv Appl Bioinform Chem. 2024 Sep 23;17:83-105. doi: 10.2147/AABC.S476404. eCollection 2024. Adv Appl Bioinform Chem. 2024. PMID: 39345873 Free PMC article.
-
Development and experimental validation of a machine learning model for the prediction of new antimalarials.BMC Chem. 2025 Jan 30;19(1):28. doi: 10.1186/s13065-025-01395-4. BMC Chem. 2025. PMID: 39885590 Free PMC article.
References
-
- World Health Organization World Malaria Report 2019. World Health Organization; Geneva, Switzerland: 2019.
-
- World Health Organization. Global Malaria Programme . Global Technical Strategy for Malaria, 2016–2030. World Health Organization; Geneva, Switzerland: 2015.
-
- Birnbaum J., Scharf S., Schmidt S., Jonscher E., Hoeijmakers W.A.M., Flemming S., Toenhake C.G., Schmitt M., Sabitzki R., Bergmann B., et al. A Kelch13-defined endocytosis pathway mediates artemisinin resistance in malaria parasites. Science. 2020;367:51–59. doi: 10.1126/science.aax4735. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

