Klotho Deficiency Causes Heart Aging via Impairing the Nrf2-GR Pathway
- PMID: 33334122
- PMCID: PMC8782577
- DOI: 10.1161/CIRCRESAHA.120.317348
Klotho Deficiency Causes Heart Aging via Impairing the Nrf2-GR Pathway
Erratum in
-
Correction to: Klotho Deficiency Causes Heart Aging via Impairing the Nrf2-GR Pathway.Circ Res. 2025 Sep 12;137(7):e175. doi: 10.1161/RES.0000000000000730. Epub 2025 Sep 11. Circ Res. 2025. PMID: 40934297 No abstract available.
Abstract
Rationale: Cardiac aging is an important contributing factor for heart failure, which affects a large population but remains poorly understood.
Objective: The purpose of this study is to investigate whether Klotho plays a role in cardiac aging.
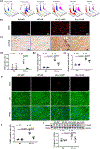
Methods and results: Heart function declined in old mice (24 months), as evidenced by decreases in fractional shortening, ejection fraction, and cardiac output. Heart size and weight, cardiomyocyte size, and cardiac fibrosis were increased in old mice, indicating that aging causes cardiac hypertrophy and remodeling. Circulating Klotho levels were dramatically decreased in old mice, which prompted us to investigate whether the Klotho decline may cause heart aging. We found that Klotho gene mutation (KL-/-) largely decreased serum klotho levels and impaired heart function. Interestingly, supplement of exogenous secreted Klotho prevented heart failure, hypertrophy, and remodeling in both old mice and KL (-/-) mice. Secreted Klotho treatment inhibited excessive cardiac oxidative stress, senescence and apoptosis in old mice and KL (-/-) mice. Serum phosphate levels in KL (-/-) mice were kept in the normal range, suggesting that Klotho deficiency-induced heart aging is independent of phosphate metabolism. Mechanistically, Klotho deficiency suppressed GR (glutathione reductase) expression and activity in the heart via inhibition of transcription factor Nrf2 (nuclear factor-erythroid 2 p45-related factor 2). Furthermore, cardiac-specific overexpression of GR prevented excessive oxidative stress, apoptosis, and heart failure in both old and KL (-/-) mice.
Conclusions: Klotho deficiency causes cardiac aging via impairing the Nrf2-GR pathway. Supplement of exogenous secreted Klotho represents a promising therapeutic strategy for aging-associated cardiomyopathy and heart failure.
Keywords: aging; apoptosis; glutathione reductase; heart failure; oxidative stress.
Figures





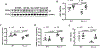

Comment in
-
Demystifying Cardiac Aging: Still Much to Learn.Circ Res. 2021 Feb 19;128(4):508-510. doi: 10.1161/CIRCRESAHA.121.318741. Epub 2021 Feb 18. Circ Res. 2021. PMID: 33600232 No abstract available.
References
-
- Hayflick L The future of ageing. Nature. 2000;408:267–9. - PubMed
-
- Hayflick L New approaches to old age. Nature. 2000;403:365. - PubMed
-
- Tepp K, Timohhina N, Puurand M, Klepinin A, Chekulayev V, Shevchuk I and Kaambre T. Bioenergetics of the aging heart and skeletal muscles: Modern concepts and controversies. Ageing research reviews. 2016;28:1–14. - PubMed
-
- McMurray JJ and Pfeffer MA. Heart failure. Lancet. 2005;365:1877–89. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

