Broad cross-national public support for accelerated COVID-19 vaccine trial designs
- PMID: 33334616
- PMCID: PMC7831807
- DOI: 10.1016/j.vaccine.2020.11.072
Broad cross-national public support for accelerated COVID-19 vaccine trial designs
Abstract
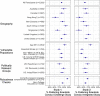
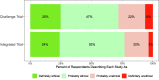
A vaccine for COVID-19 is urgently needed. Several vaccine trial designs may significantly accelerate vaccine testing and approval, but also increase risks to human subjects. Concerns about whether the public would see such designs as ethical represent an important roadblock to their implementation; accordingly, both the World Health Organization and numerous scholars have called for consulting the public regarding them. We answered these calls by conducting a cross-national survey (n = 5920) in Australia, Canada, Hong Kong, New Zealand, South Africa, Singapore, the United Kingdom, and the United States. The survey explained key differences between traditional vaccine trials and two accelerated designs: a challenge trial or a trial integrating a Phase II safety and immunogenicity trial into a larger Phase III efficacy trial. Respondents' answers to comprehension questions indicate that they largely understood the key differences and ethical trade-offs between the designs from our descriptions. We asked respondents whether they would prefer scientists to conduct traditional trials or one of these two accelerated designs. We found broad majorities prefer for scientists to conduct challenge trials (75%) and integrated trials (63%) over standard trials. Even as respondents acknowledged the risks, they perceived both accelerated trials as similarly ethical to standard trial designs. This high support is consistent across every geography and demographic subgroup we examined, including vulnerable populations. These findings may help assuage some of the concerns surrounding accelerated designs.
Keywords: COVID-19; Challenge trials; Public opinion; Vaccine ethics.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of Competing Interests The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

References
-
- Jackson James K., Weiss Martin A., Schwarzenberg Andres B., Nelson Rebecca M. Global economic effects of covid-19. Technical report. 2020
-
- World Health Organization. Draft landscape of covid-19 candidate vaccines. Available at https://www.who.int/who-documents-detail/draft-landscape-of-covid-19-can..., July 2020a.
-
- Liu Qing, Pledger Gordon W. Phase 2 and 3 combination designs to accelerate drug development. J. Am. Stat. Assoc. 2005;100(470):493–502.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

