Cancer-Associated Neurogenesis and Nerve-Cancer Cross-talk
- PMID: 33334813
- PMCID: PMC7969424
- DOI: 10.1158/0008-5472.CAN-20-2793
Cancer-Associated Neurogenesis and Nerve-Cancer Cross-talk
Abstract
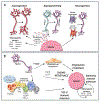
In this review, we highlight recent discoveries regarding mechanisms contributing to nerve-cancer cross-talk and the effects of nerve-cancer cross-talk on tumor progression and dissemination. High intratumoral nerve density correlates with poor prognosis and high recurrence across multiple solid tumor types. Recent research has shown that cancer cells express neurotrophic markers such as nerve growth factor, brain-derived neurotrophic factor, and glial cell-derived neurotrophic factor and release axon-guidance molecules such as ephrin B1 to promote axonogenesis. Tumor cells recruit new neural progenitors to the tumor milieu and facilitate their maturation into adrenergic infiltrating nerves. Tumors also rewire established nerves to adrenergic phenotypes via exosome-induced neural reprogramming by p53-deficient tumors. In turn, infiltrating sympathetic nerves facilitate cancer progression. Intratumoral adrenergic nerves release noradrenaline to stimulate angiogenesis via VEGF signaling and enhance the rate of tumor growth. Intratumoral parasympathetic nerves may have a dichotomous role in cancer progression and may induce Wnt-β-catenin signals that expand cancer stem cells. Importantly, infiltrating nerves not only influence the tumor cells themselves but also impact other cells of the tumor stroma. This leads to enhanced sympathetic signaling and glucocorticoid production, which influences neutrophil and macrophage differentiation, lymphocyte phenotype, and potentially lymphocyte function. Although much remains unexplored within this field, fundamental discoveries underscore the importance of nerve-cancer cross-talk to tumor progression and may provide the foundation for developing effective targets for the inhibition of tumor-induced neurogenesis and tumor progression.
©2020 American Association for Cancer Research.
Conflict of interest statement
Conflict of interest disclosure statement: The authors declare no conflicts of interest.
Figures
References
-
- Bapat AA, Hostetter G, Von Hoff DD, Han H. Perineural invasion and associated pain in pancreatic cancer. Nat Rev Cancer. 2011;11:695–707. - PubMed
-
- Deshmukh SD, Willmann JK, Jeffrey RB. Pathways of extrapancreatic perineural invasion by pancreatic adenocarcinoma: Evaluation with 3D volume-rendered MDCT imaging. Am J Roentgenol. 2010;194:668–74. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

