The antiretroviral 2',3'-dideoxycytidine causes mitochondrial dysfunction in proliferating and differentiated HepaRG human cell cultures
- PMID: 33334881
- PMCID: PMC7948951
- DOI: 10.1074/jbc.RA120.014885
The antiretroviral 2',3'-dideoxycytidine causes mitochondrial dysfunction in proliferating and differentiated HepaRG human cell cultures
Abstract
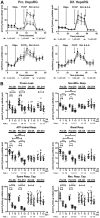
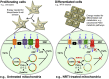
Nucleoside reverse transcriptase inhibitors (NRTIs) were the first drugs used to treat human immunodeficiency virus infection, and their use can cause mitochondrial toxicity, including mitochondrial DNA (mtDNA) depletion in several cases. The first-generation NRTIs, including 2',3'-dideoxycytidine (ddC), were originally and are still pursued as anticancer agents. NRTI-sensitive DNA polymerases localizing to mitochondria allow for the opportunity to poison proliferating cancer cell mtDNA replication as certain cancers rely heavily on mitochondrial functions. However, mtDNA replication is independent of the cell cycle creating a significant concern that toxicants such as ddC impair mtDNA maintenance in both proliferating and nonproliferating cells. To examine this possibility, we tested the utility of the HepaRG cell line to study ddC-induced toxicity in isogenic proliferating (undifferentiated) and nonproliferating (differentiated) cells. Following ddC exposures, we measured cell viability, mtDNA copy number, and mitochondrial bioenergetics utilizing trypan blue, Southern blotting, and extracellular flux analysis, respectively. After 13 days of 1 μM ddC exposure, proliferating and differentiated HepaRG harbored mtDNA levels of 0.9% and 17.9% compared with control cells, respectively. Cells exposed to 12 μM ddC contained even less mtDNA. By day 13, differentiated cell viability was maintained but declined for proliferating cells. Proliferating HepaRG bioenergetic parameters were severely impaired by day 8, with 1 and 12 μM ddC, whereas differentiated cells displayed defects of spare and maximal respiratory capacities (day 8) and proton-leak linked respiration (day 14) with 12 μM ddC. These results indicate HepaRG is a useful model to study proliferating and differentiated cell mitochondrial toxicant exposures.
Keywords: 2′-3′-dideoxycytidine (ddC, zalcitabine); HepaRG; bioenergetics; cell biology; human immunodeficiency virus (HIV); mitochondrial DNA (mtDNA) maintenance; mitochondrial toxicity; nucleoside reverse-transcriptase inhibitor (NRTI).
Copyright © 2020 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures




References
-
- Eggleton J.S., Nagalli S. StatPearls; Treasure Island (FL): 2020. Highly Active Antiretroviral Therapy (HAART) - PubMed
-
- Berger A.R., Arezzo J.C., Schaumburg H.H., Skowron G., Merigan T., Bozzette S., Richman D., Soo W. 2′,3′-dideoxycytidine (ddC) toxic neuropathy: a study of 52 patients. Neurology. 1993;43:358–362. - PubMed
-
- Lewis W., Copeland W.C., Day B. Mitochondrial DNA depletion, oxidative stress and mutation: mechanisms of nucleoside reverse transcriptase inhibitor toxicity. Lab. Invest. 2001;81:777–790. - PubMed
-
- Zhu C., Johansson M., Karlsson A. Incorporation of nucleoside analogs into nuclear or mitochondrial DNA is determined by the intracellular phosphorylation site. J. Biol. Chem. 2000;275:26727–26731. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

