A novel soluble complement receptor 1 fragment with enhanced therapeutic potential
- PMID: 33334893
- PMCID: PMC7948397
- DOI: 10.1074/jbc.RA120.016127
A novel soluble complement receptor 1 fragment with enhanced therapeutic potential
Abstract
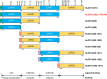
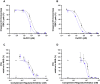
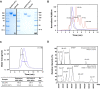
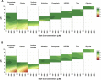
Human complement receptor 1 (HuCR1) is a pivotal regulator of complement activity, acting on all three complement pathways as a membrane-bound receptor of C3b/C4b, C3/C5 convertase decay accelerator, and cofactor for factor I-mediated cleavage of C3b and C4b. In this study, we sought to identify a minimal soluble fragment of HuCR1, which retains the complement regulatory activity of the wildtype protein. To this end, we generated recombinant, soluble, and truncated versions of HuCR1 and compared their ability to inhibit complement activation in vitro using multiple assays. A soluble form of HuCR1, truncated at amino acid 1392 and designated CSL040, was found to be a more potent inhibitor than all other truncation variants tested. CSL040 retained its affinity to both C3b and C4b as well as its cleavage and decay acceleration activity and was found to be stable under a range of buffer conditions. Pharmacokinetic studies in mice demonstrated that the level of sialylation is a major determinant of CSL040 clearance in vivo. CSL040 also showed an improved pharmacokinetic profile compared with the full extracellular domain of HuCR1. The in vivo effects of CSL040 on acute complement-mediated kidney damage were tested in an attenuated passive antiglomerular basement membrane antibody-induced glomerulonephritis model. In this model, CSL040 at 20 and 60 mg/kg significantly attenuated kidney damage at 24 h, with significant reductions in cellular infiltrates and urine albumin, consistent with protection from kidney damage. CSL040 thus represents a potential therapeutic candidate for the treatment of complement-mediated disorders.
Keywords: C3b; CSL040; complement; glomerulonephritis; glycosylation; pharmacokinetics; receptor; recombinant protein expression.
Copyright © 2020 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest S. W., H. C., A. B. M., T. R., and M. P. H. are listed as inventors on International Patent Publication number WO2019/218009. All authors with the exception of A. G. N., G. A. P., G. M.- R., M. M., and M. S. are CSL shareholders.
Figures



Similar articles
-
Sialylation-dependent pharmacokinetics and differential complement pathway inhibition are hallmarks of CR1 activity in vivo.Biochem J. 2022 May 13;479(9):1007-1030. doi: 10.1042/BCJ20220054. Biochem J. 2022. PMID: 35470373
-
Mechanistic insights into complement pathway inhibition by CR1 domain duplication.J Biol Chem. 2024 Jul;300(7):107451. doi: 10.1016/j.jbc.2024.107451. Epub 2024 Jun 4. J Biol Chem. 2024. PMID: 38844131 Free PMC article.
-
A potent truncated form of human soluble CR1 is protective in a mouse model of renal ischemia-reperfusion injury.Sci Rep. 2021 Nov 8;11(1):21873. doi: 10.1038/s41598-021-01423-y. Sci Rep. 2021. PMID: 34750424 Free PMC article.
-
The Molecular Mechanisms of Complement Receptor 1-It Is Complicated.Biomolecules. 2023 Oct 13;13(10):1522. doi: 10.3390/biom13101522. Biomolecules. 2023. PMID: 37892204 Free PMC article. Review.
-
Structure-function relationships of complement receptor type 1.Immunol Rev. 2001 Apr;180:112-22. doi: 10.1034/j.1600-065x.2001.1800110.x. Immunol Rev. 2001. PMID: 11414353 Review.
Cited by
-
Complement-targeting therapeutics for ischemia-reperfusion injury in transplantation and the potential for ex vivo delivery.Front Immunol. 2022 Oct 19;13:1000172. doi: 10.3389/fimmu.2022.1000172. eCollection 2022. Front Immunol. 2022. PMID: 36341433 Free PMC article. Review.
-
Monocyte uptake of polymeric peptidoglycan is bimodal and governed by complement C3 and C4 opsonins.JCI Insight. 2024 Dec 10;10(2):e186346. doi: 10.1172/jci.insight.186346. JCI Insight. 2024. PMID: 39656526 Free PMC article.
-
Protein therapeutics and their lessons: Expect the unexpected when inhibiting the multi-protein cascade of the complement system.Immunol Rev. 2023 Jan;313(1):376-401. doi: 10.1111/imr.13164. Epub 2022 Nov 18. Immunol Rev. 2023. PMID: 36398537 Free PMC article. Review.
-
Complement in human disease: approved and up-and-coming therapeutics.Lancet. 2024 Jan 27;403(10424):392-405. doi: 10.1016/S0140-6736(23)01524-6. Epub 2023 Nov 15. Lancet. 2024. PMID: 37979593 Free PMC article. Review.
-
A new Drosophila model of prolonged inactivity shortens lifespan and impairs muscle function.Sci Rep. 2025 Jul 31;15(1):27908. doi: 10.1038/s41598-025-13446-w. Sci Rep. 2025. PMID: 40745192 Free PMC article.
References
-
- Kolev M., Le Friec G., Kemper C. Complement--tapping into new sites and effector systems. Nat. Rev. Immunol. 2014;14:811–820. - PubMed
-
- Lachmann P.J. The amplification loop of the complement pathways. Adv. Immunol. 2009;104:115–149. - PubMed
-
- Liu D., Niu Z.X. The structure, genetic polymorphisms, expression and biological functions of complement receptor type 1 (CR1/CD35) Immunopharmacol. Immunotoxicol. 2009;31:524–535. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

