A randomised controlled trial of the effect of a connected inhaler system on medication adherence in uncontrolled asthmatic patients
- PMID: 33334936
- PMCID: PMC8176348
- DOI: 10.1183/13993003.03103-2020
A randomised controlled trial of the effect of a connected inhaler system on medication adherence in uncontrolled asthmatic patients
Abstract
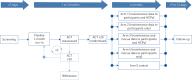
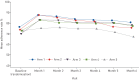
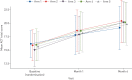
Suboptimal adherence to maintenance therapy contributes to poor asthma control and exacerbations. This study evaluated the effect of different elements of a connected inhaler system (CIS), comprising clip-on inhaler sensors, a patient-facing app and a healthcare professional (HCP) dashboard, on adherence to asthma maintenance therapy.This was an open-label, parallel-group, 6-month, randomised controlled trial in adults with uncontrolled asthma (asthma control test (ACT) score less than 20) on fixed-dose inhaled corticosteroids/long-acting β-agonist maintenance therapy (n=437). All subjects received fluticasone furoate/vilanterol ELLIPTA dry-powder inhalers for maintenance and salbutamol/albuterol metered-dose inhalers for rescue, with a sensor attached to each inhaler. Participants were randomised to one of five CIS study arms (allocation ratio 1:1:1:1:1) reflecting the recipient of the data feedback from the sensors, as follows: 1) maintenance use to participants and HCPs (n=87); 2) maintenance use to participants (n=88); 3) maintenance and rescue use to participants and HCPs (n=88); 4) maintenance and rescue use to participants (n=88); and 5) no feedback (control) (n=86).For the primary endpoint, observed mean±sd adherence to maintenance therapy over months 4-6 was 82.2±16.58% (n=83) in the "maintenance to participants and HCPs" arm and 70.8±27.30% (n=85) in the control arm. The adjusted least squares mean±se was 80.9±3.19% and 69.0±3.19%, respectively (study arm difference: 12.0%, 95% CI 5.2-18.8%; p<0.001). Adherence was also significantly greater in the other CIS arms versus the control arm. The mean percentage of rescue medication free days (months 4-6) was significantly greater in participants receiving data on their rescue use compared with controls. ACT scores improved in all study arms with no significant differences between groups.A CIS can improve adherence to maintenance medication and reduce rescue medication use in patients with uncontrolled asthma.
Trial registration: ClinicalTrials.gov NCT03380429.
Copyright ©ERS 2021.
Conflict of interest statement
Conflict of interest: A. Moore reports study funding and non-financial (writing) support from GlaxoSmithKline, during the conduct of the study; and is an employee of and holds shares/options in GlaxoSmithKline. Conflict of interest: A. Preece reports study funding and non-financial (writing) support from GlaxoSmithKline, during the conduct of the study; and is an employee of and holds shares/options in GlaxoSmithKline. Conflict of interest: R. Sharma reports study funding and non-financial (writing) support from GlaxoSmithKline, during the conduct of the study; and is an employee of and holds shares/options in GlaxoSmithKline. Conflict of interest: L.G. Heaney is lead for the UK MRC Consortium for Stratified Medicine in Severe Asthma with Amgen, AstraZeneca, Medimmune, Janssen, Novartis, Roche/Genentech, GlaxoSmithKline plc., Boehringer Ingelheim, Aerocrine and Vitalograph as industry partners; has received support for advisory boards/lectures from Novartis, Hoffman la Roche/Genentech Inc, Evelo Biosciences, Sanofi, GlaxoSmithKline, AstraZeneca, Teva, Theravance and Circassia; has received travel funding support for international respiratory meeting attendance (institution remunerated) from AstraZeneca, Boehringer Ingelheim, Chiesi, GSK and Napp Pharmaceutical; project grant funding from Medimmune, Novartis UK, Roche/Genentech, and GlaxoSmithKline plc.; and has participated in clinical trials for which their institution was remunerated from AstraZeneca, GlaxoSmithKline, Schering Plough, Synairgen, Novartis and Roche/Genentech, outside the submitted work. Conflict of interest: R.W. Costello reports grants and personal fees from GSK and Aerogen; personal fees from Novartis and TEVA; and grants from Therevance, outside the submitted work; they have patents related to acoustic measures to assess inhaler technique and methods to quantify adherence. Conflict of interest: R.A. Wise reports personal fees for consultancy from GlaxoSmithKline (GSK), during the conduct of the study; grants and personal fees for data monitoring committee work from AstraZeneca, Medimmune and Pearl; grants and personal fees for data monitoring committee and steering committee work from Boehringer Ingelheim; personal fees for steering committee work from Contrafect, Spiration, Kiniksa and Bristol Myers Squibb; personal fees for data monitoring committee work from Pulmonx, Roche, Merck, AbbVie and Kamada; personal fees for consultancy and workshops from Sunovion; grants from Pearl Therapeutics and Sanofi-Aventis; personal fees for consultancy from Circassia, Pneuma, Verona, Mylan/Theravance, Propeller Health and Novartis; grants and personal fees for data monitoring committee work, advisory board work, steering committee work and consultancy from GSK, outside the submitted work. Conflict of interest: A. Ludwig-Sengpiel has nothing to disclose. Conflict of interest: G. Mosnaim reports grants and other fees (consultant/advisory board) from GlaxoSmithKline plc.; grants and other fees (consultant and/or member of a scientific advisory board) from Propeller Health and AstraZeneca; other fees (consultant and/or member of a scientific advisory board) from Sanofi-Regeneron, Teva, Novartis and Boehringer Ingelheim; grants and other fees from AstraZeneca, outside the submitted work; they own stock in Electrocore. Conflict of interest: J. Rees reports study funding and non-financial (writing) support from GlaxoSmithKline, during the conduct of the study; and is an employee of and holds shares/options in GlaxoSmithKline. Conflict of interest: R. Tomlinson reports study funding and non-financial (writing) support from GlaxoSmithKline, during the conduct of the study; and is an employee of and holds shares/options in GlaxoSmithKline. Conflict of interest: R. Tal-Singer reports study funding and non-financial (writing) support from GlaxoSmithKline, during the conduct of the study; and is a former employee of and holds shares/options in GlaxoSmithKline. Conflict of interest: D.A. Stempel reports study funding and non-financial (writing) support from GlaxoSmithKline, during the conduct of the study; is a former employee of and holds shares/options in GlaxoSmithKline; and is an employee of and holds shares/options in Propeller Health. Conflict of interest: N. Barnes reports study funding and non-financial (writing) support from GlaxoSmithKline, during the conduct of the study; and is an employee of and holds shares/options in GlaxoSmithKline.
Figures

Comment in
-
Improving adherence to asthma medications: same-old, same-old?Eur Respir J. 2021 Jun 4;57(6):2004284. doi: 10.1183/13993003.04284-2020. Print 2021 Jun. Eur Respir J. 2021. PMID: 34088756 No abstract available.
References
-
- GBD 2015 Chronic Respiratory Disease Collaborators. Global, regional, and national deaths, prevalence, disability-adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Respir Med 2017; 5: 691–706. - PMC - PubMed
-
- Global Initiative for Asthma. Global strategy for asthma management and prevention, 2020. https://ginasthma.org/wp-content/uploads/2020/06/GINA-2020-report_20_06_.... Date last updated: 2020. Date last accessed: November 05, 2020.
-
- Engelkes M, Janssens HM, de Jongste JC, et al. Medication adherence and the risk of severe asthma exacerbations: a systematic review. Eur Respir J 2015; 45: 396–407. - PubMed
-
- Haughney J, Price D, Kaplan A, et al. Achieving asthma control in practice: understanding the reasons for poor control. Respir Med 2008; 102: 1681–1693. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
