Nonhuman primate to human immunobridging to infer the protective effect of an Ebola virus vaccine candidate
- PMID: 33335092
- PMCID: PMC7747701
- DOI: 10.1038/s41541-020-00261-9
Nonhuman primate to human immunobridging to infer the protective effect of an Ebola virus vaccine candidate
Abstract
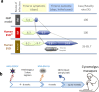
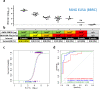
It has been proven challenging to conduct traditional efficacy trials for Ebola virus (EBOV) vaccines. In the absence of efficacy data, immunobridging is an approach to infer the likelihood of a vaccine protective effect, by translating vaccine immunogenicity in humans to a protective effect, using the relationship between vaccine immunogenicity and the desired outcome in a suitable animal model. We here propose to infer the protective effect of the Ad26.ZEBOV, MVA-BN-Filo vaccine regimen with an 8-week interval in humans by immunobridging. Immunogenicity and protective efficacy data were obtained for Ad26.ZEBOV and MVA-BN-Filo vaccine regimens using a fully lethal EBOV Kikwit challenge model in cynomolgus monkeys (nonhuman primates [NHP]). The association between EBOV neutralizing antibodies, glycoprotein (GP)-binding antibodies, and GP-reactive T cells and survival in NHP was assessed by logistic regression analysis. Binding antibodies against the EBOV surface GP were identified as the immune parameter with the strongest correlation to survival post EBOV challenge, and used to infer the predicted protective effect of the vaccine in humans using published data from phase I studies. The human vaccine-elicited EBOV GP-binding antibody levels are in a range associated with significant protection against mortality in NHP. Based on this immunobridging analysis, the EBOV GP-specific-binding antibody levels elicited by the Ad26.ZEBOV, MVA-BN-Filo vaccine regimen in humans will likely provide protection against EBOV disease.
Conflict of interest statement
The authors declare the following competing interests: RR, JH, TvE, BS, LD, LS, DC-C, VB, JSadoff, SJ, NV, JSerroyen, ED, CR, ML, MD, KL, MGP, JStoop, AVandebosch, HS, RZ, and BC are employed by Janssen Pharmaceuticals and may hold stock in Johnson & Johnson. AVollkman is an employee and may hold stock in Bavarian Nordic. NV is employed and may hold stock in Galapagos NV. All other authors have nothing to disclose.
Figures



References
-
- FDA. Product Development Under the Animal Rule (FDA, 2015). https://www.fda.gov/media/88625/download.
-
- EMA. Conditional Marketing Authorisation (EMA, 2006). https://www.ema.europa.eu/en/human-regulatory/marketing-authorisation/co....
-
- EMA. Guideline on Procedures for the Granting of a Marketing Authorization Under Exceptional Circumstances, Pursuant to Article 14 (8) of Regulation (EC) no. 726/2004 (EMA, 2005). https://www.ema.europa.eu/en/documents/regulatory-procedural-guideline/g....
Grants and funding
LinkOut - more resources
Full Text Sources

