A systematic review and meta-analysis on chloroquine and hydroxychloroquine as monotherapy or combined with azithromycin in COVID-19 treatment
- PMID: 33335141
- PMCID: PMC7746770
- DOI: 10.1038/s41598-020-77748-x
A systematic review and meta-analysis on chloroquine and hydroxychloroquine as monotherapy or combined with azithromycin in COVID-19 treatment
Abstract
Many recent studies have investigated the role of either Chloroquine (CQ) or Hydroxychloroquine (HCQ) alone or in combination with azithromycin (AZM) in the management of the emerging coronavirus. This systematic review and meta-analysis of either published or preprint observational studies or randomized control trials (RCT) aimed to assess mortality rate, duration of hospital stay, need for mechanical ventilation (MV), virologic cure rate (VQR), time to a negative viral polymerase chain reaction (PCR), radiological progression, experiencing drug side effects, and clinical worsening. A search of the online database through June 2020 was performed and examined the reference lists of pertinent articles for in-vivo studies only. Pooled relative risks (RRs), standard mean differences of 95% confidence intervals (CIs) were calculated with the random-effects model. Mortality was not different between the standard care (SC) and HCQ groups (RR = 0.99, 95% CI 0.61-1.59, I2 = 82%), meta-regression analysis proved that mortality was significantly different across the studies from different countries. However, mortality among the HCQ + AZM was significantly higher than among the SC (RR = 1.8, 95% CI 1.19-2.27, I2 = 70%). The duration of hospital stay in days was shorter in the SC in comparison with the HCQ group (standard mean difference = 0.57, 95% CI 0.20-0.94, I2 = 92%), or the HCQ + AZM (standard mean difference = 0.77, 95% CI 0.46-1.08, I2 = 81). Overall VQR, and that at days 4, 10, and 14 among patients exposed to HCQ did not differ significantly from the SC [(RR = 0.92, 95% CI 0.69-1.23, I2 = 67%), (RR = 1.11, 95% CI 0.26-4.69, I2 = 85%), (RR = 1.21, 95% CI 0.70-2.01, I2 = 95%), and (RR = 0.98, 95% CI 0.76-1.27, I2 = 85% )] respectively. Exposure to HCQ + AZM did not improve the VQR as well (RR = 3.23, 95% CI 0.70-14.97, I2 = 58%). The need for MV was not significantly different between the SC and HCQ (RR = 1.5, 95% CI 0.78-2.89, I2 = 81%), or HCQ + AZM (RR = 1.27, 95% CI 0.7-2.13, I2 = 88%). Side effects were more reported in the HCQ group than in the SC (RR = 3.14, 95% CI 1.58-6.24, I2 = 0). Radiological improvement and clinical worsening were not statistically different between HCQ and SC [(RR = 1.11, 95% CI 0.74-1.65, I2 = 45%) and (RR = 1.28, 95% CI 0.33-4.99), I2 = 54%] respectively. Despite the scarcity of published data of good quality, the effectiveness and safety of either HCQ alone or in combination with AZM in treating COVID-19 cannot be assured. Future high-quality RCTs need to be carried out.PROSPERO registration: CRD42020192084.
Conflict of interest statement
The authors declare no competing interests.
Figures
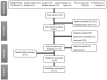


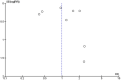





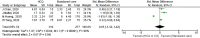
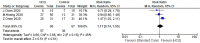
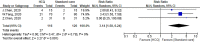

References
-
- World meter. COVID-19 Coronavirus Pandemic, https://www.worldometers.info/coronavirus/ (2020).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

