Lipid yield from the diatom Porosira glacialis is determined by solvent choice and number of extractions, independent of cell disruption
- PMID: 33335240
- PMCID: PMC7747635
- DOI: 10.1038/s41598-020-79269-z
Lipid yield from the diatom Porosira glacialis is determined by solvent choice and number of extractions, independent of cell disruption
Abstract
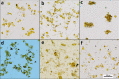
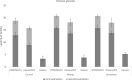
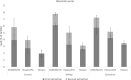
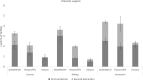
Cell wall disruption is necessary to maximize lipid extraction yields in conventional species of mass-cultivated microalgae. This study investigated the effect of sonication, solvent choice and number of extractions on the lipid yield, lipid class composition and fatty acid composition of the diatom Porosira glacialis. For comparison, the diatom Odontella aurita and green alga Chlorella vulgaris were included in the study. Sonication effectively disrupted P. glacialis cells, but did not increase the total lipid yield compared to physical stirring (mixing). In all three microalgae, the content of membrane-associated glyco- and phosopholipids in the extracted lipids was strongly dependent on the solvent polarity. A second extraction resulted in higher yields from the microalgae only when polar solvents were used. In conclusion, choice of solvent and number of extractions were the main factors that determined lipid yield and lipid class composition in P. glacialis.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Borowitzka MA. High-value products from microalgae-their development and commercialisation. J. Appl. Phycol. 2013;25:743–756. doi: 10.1007/s10811-013-9983-9. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

