Bone marrow stromal cell therapy improves survival after radiation injury but does not restore endogenous hematopoiesis
- PMID: 33335275
- PMCID: PMC7747726
- DOI: 10.1038/s41598-020-79278-y
Bone marrow stromal cell therapy improves survival after radiation injury but does not restore endogenous hematopoiesis
Erratum in
-
Publisher Correction: Bone marrow stromal cell therapy improves survival after radiation injury but does not restore endogenous hematopoiesis.Sci Rep. 2021 Aug 4;11(1):16225. doi: 10.1038/s41598-021-95556-9. Sci Rep. 2021. PMID: 34349217 Free PMC article. No abstract available.
Abstract
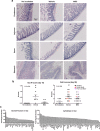
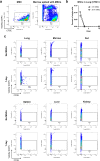
The only available option to treat radiation-induced hematopoietic syndrome is allogeneic hematopoietic cell transplantation, a therapy unavailable to many patients undergoing treatment for malignancy, which would also be infeasible in a radiological disaster. Stromal cells serve as critical components of the hematopoietic stem cell niche and are thought to protect hematopoietic cells under stress. Prior studies that have transplanted mesenchymal stromal cells (MSCs) without co-administration of a hematopoietic graft have shown underwhelming rescue of endogenous hematopoiesis and have delivered the cells within 24 h of radiation exposure. Herein, we examine the efficacy of a human bone marrow-derived MSC therapy delivered at 3 h or 30 h in ameliorating radiation-induced hematopoietic syndrome and show that pancytopenia persists despite MSC therapy. Animals exposed to radiation had poorer survival and experienced loss of leukocytes, platelets, and red blood cells. Importantly, mice that received a therapeutic dose of MSCs were significantly less likely to die but experienced equivalent collapse of the hematopoietic system. The cause of the improved survival was unclear, as complete blood counts, splenic and marrow cellularity, numbers and function of hematopoietic stem and progenitor cells, and frequency of niche cells were not significantly improved by MSC therapy. Moreover, human MSCs were not detected in the bone marrow. MSC therapy reduced crypt dropout in the small intestine and promoted elevated expression of growth factors with established roles in gut development and regeneration, including PDGF-A, IGFBP-3, IGFBP-2, and IGF-1. We conclude that MSC therapy improves survival not through overt hematopoietic rescue but by positive impact on other radiosensitive tissues, such as the intestinal mucosa. Collectively, these data reveal that MSCs could be an effective countermeasure in cancer patients and victims of nuclear accidents but that MSCs alone do not significantly accelerate or contribute to recovery of the blood system.
Conflict of interest statement
B.S.G., C.S.C., and P.L.W. are inventors on a patent for conditioning of stem and progenitor cells for cellular therapy (U.S. Patent US20180187141A1). All other authors declare no conflict of interest.
Figures






References
-
- Mauch P, et al. Hematopoietic stem cell compartment: acute and late effects of radiation therapy and chemotherapy. Int. J. Radiat. Oncol. Biol. Phys. 1995;31:1319–1339. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

