COVID-19: The Emerging Immunopathological Determinants for Recovery or Death
- PMID: 33335518
- PMCID: PMC7736111
- DOI: 10.3389/fmicb.2020.588409
COVID-19: The Emerging Immunopathological Determinants for Recovery or Death
Abstract
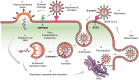
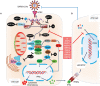
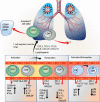
Hyperactivation of the host immune system during infection by SARS-CoV-2 is the leading cause of death in COVID-19 patients. It is also evident that patients who develop mild/moderate symptoms and successfully recover display functional and well-regulated immune response. Whereas a delayed initial interferon response is associated with severe disease outcome and can be the tipping point towards immunopathological deterioration, often preceding death in COVID-19 patients. Further, adaptive immune response during COVID-19 is heterogeneous and poorly understood. At the same time, some studies suggest activated T and B cell response in severe and critically ill patients and the presence of SARS-CoV2-specific antibodies. Thus, understanding this problem and the underlying molecular pathways implicated in host immune function/dysfunction is imperative to devise effective therapeutic interventions. In this comprehensive review, we discuss the emerging immunopathological determinants and the mechanism of virus evasion by the host cell immune system. Using the knowledge gained from previous respiratory viruses and the emerging clinical and molecular findings on SARS-CoV-2, we have tried to provide a holistic understanding of the host innate and adaptive immune response that may determine disease outcome. Considering the critical role of the adaptive immune system during the viral clearance, we have presented the molecular insights of the plausible mechanisms involved in impaired T cell function/dysfunction during various stages of COVID-19.
Keywords: COVID-19; SARS-CoV-2; T cell response; interferon response; lymphocytopaenia; viral evasion.
Copyright © 2020 Ahmad, Chaudhuri, Joshi, Almatroudi, Rahmani and Ali.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Anderson J., Schauer J., Bryant S., Graves C. R. (2020). The use of convalescent plasma therapy and remdesivir in the successful management of a critically ill obstetric patient with novel coronavirus 2019 infection: a case report. Case Rep. Womens Heal. 27:e00221. 10.1016/j.crwh.2020.e00221 - DOI - PMC - PubMed
-
- Anft M., Paniskaki K., Blazquez-Navarro A., Doevelaar A. A. N., Seibert F., Hoelzer B., et al. (2020). COVID-19 progression is potentially driven by T cell immunopathogenesis. medRxiv [Preprint] 10.1101/2020.04.28.20083089 - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

