Permeation mechanisms through the selectivity filter and the open helix bundle crossing gate of GIRK2
- PMID: 33335691
- PMCID: PMC7734222
- DOI: 10.1016/j.csbj.2020.11.039
Permeation mechanisms through the selectivity filter and the open helix bundle crossing gate of GIRK2
Abstract
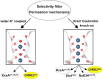
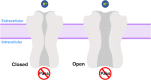
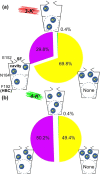
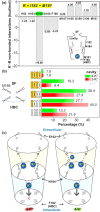
G protein-gated inwardly rectifying potassium channels (GIRK) are essential for the regulation of cellular excitability, a physiological function that relies critically on the conduction of K+ ions, which is dependent on two molecular mechanisms, namely selectivity and gating. Molecular Dynamics (MD) studies have shown that K+ conduction remains inefficient even with open channel gates, therefore further detailed study on the permeation events is required. In this study, all-atom MD simulations were employed to investigate the permeation mechanism through the GIRK2 selectivity filter (SF) and its open helix bundle crossing (HBC) gate. Our results show that it is the SF rather than the HBC or the G-loop gate that determines the permeation efficiency upon activation of the channel. SF-permeation is accomplished by a water-K+ coupled mechanism and the entry to the S1 coordination site is likely affected by a SF tilt. Moreover, we show that a 4-K+ occupancy in the SF-HBC cavity is required for the permeation through an open HBC, where three K+ ions around E152 help to abolish the unfavorable cation-dipole interactions that function as an energy barrier, while the fourth K+ located near the HBC allows for the inward transport. These findings facilitate further understanding of the dynamic permeation mechanisms through GIRK2 and potentially provide an alternative regulatory approach for the Kir3 family given the overall high evolutionary residue conservation.
Keywords: G protein-gated inwardly rectifying K+ 2 (GIRK2), permeation mechanism; Helix bundle crossing gate; Inward rectifier potassium (Kir) channel; Molecular dynamics.
© 2020 The Author(s).
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures






References
LinkOut - more resources
Full Text Sources
