Impact of crosslink heterogeneity on extracellular matrix mechanics and remodeling
- PMID: 33335693
- PMCID: PMC7734217
- DOI: 10.1016/j.csbj.2020.11.038
Impact of crosslink heterogeneity on extracellular matrix mechanics and remodeling
Abstract
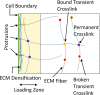
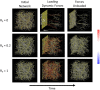
Mechanical interactions between cells and the extracellular matrix (ECM) lead to the formation of biophysical cues, notably in the form of cell-generated tension, stiffness, and concentration profiles in the ECM. Fibrillar ECMs have nonlinear stiffnesses, linked to the reorientation of fibers under stress and strain, and nonelastic properties, resulting from the force-induced unbinding of transient bonds (crosslinks) that interconnect fibers. Mechanical forces generated by cells can lead to local ECM stiffening and densification. Cell tension is also propagated through the ECM network. The underlying factors that regulate the relative emergence of these signals are not well understood. Here, through computational simulations of 3D ECM fiber networks, we show that the composition of ECM crosslinks is a key determinant of the degree of densification and stiffening that can be achieved by cell-generated forces. This also regulates the sustainability of tensions propagated through the ECM. In particular, highly transient force-sensitive crosslinks promote nonelastic densification and rapid tension relaxation, whereas permanent crosslinks promote nonlinear stiffening and stable tension profiles. A heterogeneous population of crosslinks with different unbinding kinetics enables ECMs to exhibit accumulation, tension propagation, and stiffening simultaneously in response to mechanical interactions with cells.
Keywords: Cell-matrix interactions; Computational modeling; Crosslinking; Extracellular matrix; Mechanobiology.
© 2020 The Author(s).
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures





References
Grants and funding
LinkOut - more resources
Full Text Sources
