Tongxinluo promotes axonal plasticity and functional recovery after stroke
- PMID: 33335781
- PMCID: PMC7718613
- DOI: 10.1515/tnsci-2020-0127
Tongxinluo promotes axonal plasticity and functional recovery after stroke
Erratum in
-
Corrigendum to "Tongxinluo promotes axonal plasticity and functional recovery after stroke".Transl Neurosci. 2024 Dec 21;15(1):20220988. doi: 10.1515/tnsci-2022-0988. eCollection 2024 Jan 1. Transl Neurosci. 2024. PMID: 39726893 Free PMC article.
Abstract
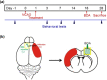
Background: The aim of this study was to investigate the neural plasticity in contralesional cortex and the effects of tongxinluo (TXL) in cerebral ischemic rats.
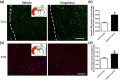
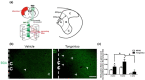
Methodology: We used stroke-prone renovascular hypertensive (RHRSP) cerebral ischemia rat models to study the effect of TXL and the underlying mechanisms. We performed foot-fault and beam-walking tests to evaluate the motor function of rats after cortical infarction. Biotinylated dextran amine (BDA) was used to track axonal sprouting and neural connections.
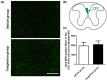
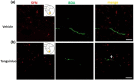
Results: TXL enhanced the recovery of motor function in cerebral infarction rats. TXL increased axonal sprouting in the peri-infarcted area but not in the corpus callosum, indicating in situ origination instead of crossing between cortical hemispheres through the corpus callosum. TXL promoted the sprouting of corticospinal axons into the denervated side of spinal gray matter. The synaptophysin (SYN)-positive intensity in the peri-infarcted area of TXL-treated group was greater than that in the vehicle group. We observed co-localization of SYN with BDA-positive fibers in the denervated spinal cord gray matter in the TXL group, suggesting that axonal remodeling and synaptic connections were promoted by TXL.
Conclusion: TXL may promote the recovery of neurological function by promoting the axonal remodeling and synapse formation of motor neuronal fibers after focal cortical infarction in hypertensive rats.
Keywords: axonal remodeling; functional recovery; biotinylated dextran amines; distal middle cerebral artery occlusion; tongxinluo.
© 2020 Xiaoting Wang et al., published by De Gruyter.
Conflict of interest statement
Conflict of interest: The authors declare that there are no conflicts of interest.
Figures


References
-
- Flack JM, Ferdinand KC, Nasser SA. Epidemiology of hypertension and cardiovascular disease in African Americans. J Clin Hypertens (Greenwich). 2003;5(Suppl 1):5–11. 10.1111/j.1524-6175.2003.02152.x. - DOI - PMC - PubMed
- Flack JM, Ferdinand KC, Nasser SA. Epidemiology of hypertension and cardiovascular disease in African Americans. J Clin Hypertens (Greenwich) 2003;5(Suppl 1):5–11. doi: 10.1111/j.1524-6175.2003.02152.x. - DOI - PMC - PubMed
-
- Joffres M, Falaschetti E, Gillespie C, Robitaille C, Loustalot F, Poulter N, et al. Hypertension prevalence, awareness, treatment and control in national surveys from England, the USA and Canada, and correlation with stroke and ischaemic heart disease mortality: A cross-sectional study. BMJ Open. 2013;3(8):e003423. 10.1136/bmjopen-2013-003423. - DOI - PMC - PubMed
- Joffres M, Falaschetti E, Gillespie C, Robitaille C, Loustalot F, Poulter N. et al. Hypertension prevalence, awareness, treatment and control in national surveys from England, the USA and Canada, and correlation with stroke and ischaemic heart disease mortality: A cross-sectional study. BMJ Open. 2013;3(8):e003423. doi: 10.1136/bmjopen-2013-003423. - DOI - PMC - PubMed
-
- Engel O, Kolodziej S, Dirnagl U, Prinz V. Modeling stroke in mice - middle cerebral artery occlusion with the filament model. J Vis Exp. 2011;47:2423. 10.3791/2423. - DOI - PMC - PubMed
- Engel O, Kolodziej S, Dirnagl U, Prinz V. Modeling stroke in mice - middle cerebral artery occlusion with the filament model. J Vis Exp. 2011;47:2423. doi: 10.3791/2423. - DOI - PMC - PubMed
-
- Zeng J, Zhang Y, Mo J, Su Z, Huang R. Two-kidney, two clip renovascular hypertensive rats can be used as stroke-prone rats. Stroke. 1998;29(8):1708–13. discussion 13–4. 10.1161/01.str.29.8.1708. - DOI - PubMed
- Zeng J, Zhang Y, Mo J, Su Z, Huang R. Two-kidney, two clip renovascular hypertensive rats can be used as stroke-prone rats. Stroke. 1998;29(8):1708–13. doi: 10.1161/01.str.29.8.1708. discussion 13–4. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
