Consolidation Immunotherapy After Platinum-Based Chemoradiotherapy in Patients With Unresectable Stage III Non-Small Cell Lung Cancer-Cross-Sectional Study of Eligibility and Administration Rates
- PMID: 33335856
- PMCID: PMC7736629
- DOI: 10.3389/fonc.2020.586449
Consolidation Immunotherapy After Platinum-Based Chemoradiotherapy in Patients With Unresectable Stage III Non-Small Cell Lung Cancer-Cross-Sectional Study of Eligibility and Administration Rates
Abstract
Introduction: The PACIFC trial demonstrated a significant benefit of durvalumab consolidation immunotherapy (CIT) after definitive platinum-based chemoradiotherapy (P-CRT) for survival in stage III non-small cell lung cancer (NSCLC). It is unknown how many patients are eligible in clinical practice to receive CIT according to PACIFIC criteria compared to real administration rates and what influencing factors are.
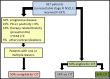
Patients and methods: We analyzed 442 patients with unresectable stage III NSCLC who received P-CRT between 2009 and 2019 regarding CIT eligibility rates according to PACIFIC criteria and administration rates since drug approval.
Results: Sixty-four percent of 437 patients were male, median age was 63 years [interquartile range (IQR): 57-69]. The most common histologic subtypes were adenocarcinoma (42.8%) and squamous cell carcinoma (41.1%), most tumors were in stage IIIB (56.8%). Mean PD-L1 tumor proportion score (TPS) was 29.8% (IQR: 1-60). The median total RT dose was 60 Gy (IQR: 60-66). Platinum component of P-CRT was evenly distributed between cisplatin (51.4%) and carboplatin (48.6%). 50.3% of patients were eligible for CIT according to PACIFIC criteria. Observed contraindications were progressive disease according to RECIST (32.4%), followed by a PD-L1 TPS < 1% (22.3%), pneumonitis CTCAE ≥ 2 (12.6%) and others (4.9%). One year after drug approval, 85.6% of patients who were eligible according to PACIFIC criteria actually received CIT. Time interval between chemotherapy start and radiation therapy start (OR 0.9, 95% CI: [0.9; 1.0] p = 0.009) and probably cisplatin as platinum-component of P-CRT (OR 1.5, 95% CI: [1.0; 2.4] p < 0.061) influence CIT eligibility. Highly positive PD-L1 TPS (≥50%; (OR 2.4, 95% CI: [1.3; 4.5] p = 0.004) was associated to a better chance for CIT eligibility.
Conclusion: Eighty-five percent of potentially eligible patients received CIT one year after drug approval. Fifty percent of patients did not meet PACIFIC criteria for durvalumab eligibility, this was mainly caused by disease progression during platinum-based CRT, followed by therapy-related pneumonitis and PD-L1 TPS < 1% (in view of the EMA drug approval).
Keywords: PACIFIC criteria; definitive platinum-based chemoradiotherapy; durvalumab admission rate; durvalumab eligibility rate; non-small cell lung cancer stage III.
Copyright © 2020 Eichkorn, Bozorgmehr, Regnery, Dinges, Kudak, Bougatf, Weber, Christopoulos, Muley, Kobinger, König, Hörner-Rieber, Adeberg, Heussel, Thomas, Debus and El Shafie.
Conflict of interest statement
TE reports grants from Ruprecht-Karls Universität Heidelberg, Herbert Kienzle Foundation, and Else Kröner-Fresenius Foundation and received travel reimbursement from Bristol-Myers Squibb outside the submitted work. JH-R received speaker fees and travel reimbursement from ViewRay Inc, as well as travel reimbursement form IntraOP Medical and Elekta Instrument AB outside the submitted work. SA acknowledges personal fees by Astra Zeneca outside the presented research work. JD reports grants from CRI The Clinical Research Institute, grants from View Ray Inc., grants from Accuray International, grants from Accuray Incorporated, grants from RaySearch Laboratories AB, grants from Vision RT limited, grants from Merck Serono GmbH, grants from Astellas Pharma GmbH, grants from Astra Zeneca GmbH, grants from Siemens Healthcare GmbH, grants from Solution Akademie GmbH, grants from Eromed PLC Surrey Research Park, grants from Quintiles GmbH, grants from Pharmaceutical Research Associates GmbH, grants from Boehringer Ingelheim Pharma GmbH Co, grants from PTW-Freiburg Dr. Pychlau GmbH, and grants from Nanobiotix A.a., outside the submitted work. RS reports grants from Ruprecht-Karls Universität Heidelberg, during the conduct of the study; personal fees from Accuray Inc., personal fees from AstraZeneca GmbH, personal fees from Bristol Myers Squibb GmbH & Co., personal fees from Novocure GmbH, personal fees from Merck KGaA, personal fees from Takeda GmbH, grants from Accuray Inc., outside the submitted work. The other authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Sakaguchi T, Ito K, Furuhashi K, Nakamura Y, Suzuki Y, Nishii Y, et al. Patients with unresectable stage III non-small cell lung cancer eligible to receive consolidation therapy with durvalumab in clinical practice based on PACIFIC study criteria. Respir Invest (2019) 57(5):466–71. 10.1016/j.resinv.2019.03.011 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

