Decreasing the Susceptibility of Malignant Cells to Infection Does Not Impact the Overall Efficacy of Myxoma Virus-Based Oncolytic Virotherapy
- PMID: 33335977
- PMCID: PMC7720075
- DOI: 10.1016/j.omto.2020.10.011
Decreasing the Susceptibility of Malignant Cells to Infection Does Not Impact the Overall Efficacy of Myxoma Virus-Based Oncolytic Virotherapy
Abstract
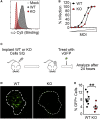
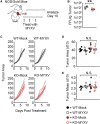
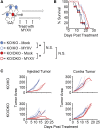
Oncolytic virotherapy relies on the induction of anti-tumor immune responses to achieve therapeutic efficacy. The factors that influence the induction of these responses, however, are not well understood. To begin to address this lack of knowledge, we asked how decreasing the susceptibility of malignant cells to direct viral infection would impact the induction of immune responses and therapeutic efficacy caused by oncolytic myxoma virus treatment. To accomplish this, we used CRISPR-Cas9 genome editing to remove the essential sulfation enzyme N-deacetylase/N-sulfotransferase-1 from B16/F10 murine melanoma cells. This eliminates the negative cell surface charges associated with glycosaminoglycan sulfation, which reduces a cell's susceptibility to infection with the myxoma virus by ∼3- to 10-fold. With the use of these cells as a model of reduced susceptibility to oncolytic infection, our data demonstrate that 3- to 10-fold reductions in in vivo infection do not hinder the ability of the oncolytic myxoma virus to induce anti-tumor immunity and do not lower the overall efficacy of localized treatment. Additionally, our data show that in mice bearing multiple distinct tumor masses, the choice to treat a less-susceptible tumor mass does not reduce the overall therapeutic impact against either the injected or noninjected lesion. Taken together, these data suggest that minor changes in the susceptibility of malignant cells to direct oncolytic infection do not necessarily influence the overall outcomes of treatment.
Keywords: immunotherapy; oncolytic virotherapy; viral dosing.
© 2020 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Nichols A.C., Yoo J., Um S., Mundi N., Palma D.A., Fung K., Macneil S.D., Koropatnick J., Mymryk J.S., Barrett J.W. Vaccinia virus outperforms a panel of other poxviruses as a potent oncolytic agent for the control of head and neck squamous cell carcinoma cell lines. Intervirology. 2014;57:17–22. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

