Bladder-embedded ectopic intrauterine device with calculus
- PMID: 33336004
- PMCID: PMC7712230
- DOI: 10.1515/med-2020-0173
Bladder-embedded ectopic intrauterine device with calculus
Erratum in
-
Erratum to "Bladder-embedded ectopic intrauterine device with calculus".Open Med (Wars). 2024 Feb 14;19(1):20231000. doi: 10.1515/med-2023-1000. eCollection 2024. Open Med (Wars). 2024. PMID: 38463524 Free PMC article.
Abstract
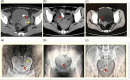
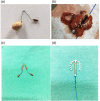
The present study aimed to analyze the data of embedded intrauterine device (IUD) in the bladder wall with the additional presence of calculus. This case series study included 11 female patients with partially or completely embedded IUD in the bladder wall. Their median age was 34 (range, 32-39) years. The median duration of IUD placement was 36 (range, 24-60) months. The median duration of symptoms was 9 (range, 3-12) months. Six patients underwent laparoscopy: the operation duration was 129 (range, 114-162) min, blood loss was 15 (range, 10-25) mL, the hospital stay was 4 (range, 4-4.5) days, the visual analog scale (VAS) for pain at 6 h after surgery was 3 (range, 2-6), and the time to removal of the urethral catheter was 7 (range, 7-8) days. Five patients underwent open surgery: the operation duration was 126 (range, 96-192) min, blood loss was 30 (range, 20-50) mL, the hospital stay was 7 (range, 7-15) days, the VAS was 6 (range, 4-9) at 6 h after surgery, and the time to removal of the urethral catheter was 9 (range, 8-17) days. The IUD and bladder stones were successfully removed in all 11 (100%) patients.
Keywords: cystectomy; intrauterine device; intrauterine device migration; laparoscopy; surgery; urinary bladder; urinary bladder calculi.
© 2020 Bing-Jian Xiong et al., published by De Gruyter.
Figures
References
-
- Espey E, Ogburn T. Long-acting reversible contraceptives: intrauterine devices and the contraceptive implant. Obstet Gynecol. 2011;117(3):705–19. 10.1097/AOG.0b013e31820ce2f0. - DOI - PubMed
- Espey E, Ogburn T. Long-acting reversible contraceptives: intrauterine devices and the contraceptive implant. Obstet Gynecol. 2011;117(3):705–19. doi: 10.1097/AOG.0b013e31820ce2f0. - DOI - PubMed
-
- Stoddard A, McNicholas C, Peipert JF. Efficacy and safety of long-acting reversible contraception. Drugs. 2011;71(8):969–80. 10.2165/11591290-000000000-00000. - DOI - PMC - PubMed
- Stoddard A, McNicholas C, Peipert JF. Efficacy and safety of long-acting reversible contraception. Drugs. 2011;71(8):969–80. doi: 10.2165/11591290-000000000-00000. - DOI - PMC - PubMed
-
- Curtis KM, Tepper NK, Jatlaoui TC, Berry-Bibee E, Horton LG, Zapata LB, et al. U.S. medical eligibility criteria for contraceptive use. MMWR Recomm Rep. 2016;65(3):1–103. 10.15585/mmwr.rr6503a1. - DOI - PubMed
- Curtis KM, Tepper NK, Jatlaoui TC, Berry-Bibee E, Horton LG, Zapata LB. et al. U.S. medical eligibility criteria for contraceptive use. MMWR Recomm Rep. 2016;65(3):1–103. doi: 10.15585/mmwr.rr6503a1. - DOI - PubMed
-
- Buhling KJ, Zite NB, Lotke P, Black K, Group IW. Worldwide use of intrauterine contraception: a review. Contraception. 2014;89(3):162–73. 10.1016/j.contraception.2013.11.011. - DOI - PubMed
- Buhling KJ, Zite NB, Lotke P, Black K, Group IW. Worldwide use of intrauterine contraception: a review. Contraception. 2014;89(3):162–73. doi: 10.1016/j.contraception.2013.11.011. - DOI - PubMed
-
- Aoun J, Dines VA, Stovall DW, Mete M, Nelson CB, Gomez-Lobo V. Effects of age, parity, and device type on complications and discontinuation of intrauterine devices. Obstet Gynecol. 2014;123(3):585–92. 10.1097/AOG.0000000000000144. - DOI - PubMed
- Aoun J, Dines VA, Stovall DW, Mete M, Nelson CB, Gomez-Lobo V. Effects of age, parity, and device type on complications and discontinuation of intrauterine devices. Obstet Gynecol. 2014;123(3):585–92. doi: 10.1097/AOG.0000000000000144. - DOI - PubMed
LinkOut - more resources
Full Text Sources
