This is a preprint.
Assessing Durability of Vaccine Effect Following Blinded Crossover in COVID-19 Vaccine Efficacy Trials
- PMID: 33336213
- PMCID: PMC7745130
- DOI: 10.1101/2020.12.14.20248137
Assessing Durability of Vaccine Effect Following Blinded Crossover in COVID-19 Vaccine Efficacy Trials
Update in
-
A Deferred-Vaccination Design to Assess Durability of COVID-19 Vaccine Effect After the Placebo Group Is Vaccinated.Ann Intern Med. 2021 Aug;174(8):1118-1125. doi: 10.7326/M20-8149. Epub 2021 Apr 13. Ann Intern Med. 2021. PMID: 33844575 Free PMC article.
Abstract
Background: Several candidate vaccines to prevent COVID-19 disease have entered large-scale phase 3 placebo-controlled randomized clinical trials and some have demonstrated substantial short-term efficacy. Efficacious vaccines should, at some point, be offered to placebo participants, which will occur before long-term efficacy and safety are known.
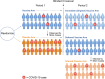
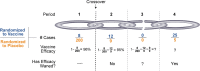
Methods: Following vaccination of the placebo group, we show that placebo-controlled vaccine efficacy can be derived by assuming the benefit of vaccination over time has the same profile for the original vaccine recipients and the placebo crossovers. This reconstruction allows estimation of both vaccine durability and potential vaccine-associated enhanced disease.
Results: Post-crossover estimates of vaccine efficacy can provide insights about durability, identify waning efficacy, and identify late enhancement of disease, but are less reliable estimates than those obtained by a standard trial where the placebo cohort is maintained. As vaccine efficacy estimates for post-crossover periods depend on prior vaccine efficacy estimates, longer pre-crossover periods with higher case counts provide better estimates of late vaccine efficacy. Further, open-label crossover may lead to riskier behavior in the immediate crossover period for the unblinded vaccine arm, confounding vaccine efficacy estimates for all post-crossover periods.
Conclusions: We advocate blinded crossover and continued follow-up of trial participants to best assess vaccine durability and potential delayed enhancement of disease. This approach allows placebo recipients timely access to the vaccine when it would no longer be proper to maintain participants on placebo, yet still allows important insights about immunological and clinical effectiveness over time.
Conflict of interest statement
Conflicts of Interest
The authors have no competing interests to declare.
Figures

References
-
- Cohen MS, Corey L. Combination prevention for COVID-19. Science 2020;368:551. - PubMed
-
- Corey L, Mascola JR, Fauci AS, Collins FS. A strategic approach to COVID-19 vaccine R&D. Science 2020;368:948–50. - PubMed
-
- Moderna. Moderna Announces Primary Efficacy Analysis in Phase 3 COVE Study for Its COVID-19 Vaccine Candidate and Filing Today with U.S. FDA for Emergency Use Authorization. November 30, 2020. press release. https://investors.modernatx.com/news-releases/news-release-details/moder.... Access date December 1, 2020.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
