Liquid Chromatographic Methods for COVID-19 Drugs, Hydroxychloroquine and Chloroquine
- PMID: 33336246
- PMCID: PMC7799265
- DOI: 10.1093/chromsci/bmaa110
Liquid Chromatographic Methods for COVID-19 Drugs, Hydroxychloroquine and Chloroquine
Abstract
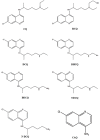
COVID-19 has been a threat throughout the world since December 2019. In attempts to discover an urgent treatment regime for COVID-19, hydroxychloroquine (HCQ) and chloroquine (CQ) have been on solidarity clinical trial. However, many countries have pulled HCQ and CQ from their COVID-19 treatment regimens recently, some countries still continue using them for patients who have previously started HCQ and CQ and they may complete their course under the supervision of a doctor. HCQ and CQ are 4-aminoquinoline drugs and it is safe to use them for autoimmune diseases, rheumatoid arthritis, systemic lupus erythematosus and malaria as well. Determination of CQ, HCQ and their metabolites in biologic fluids and in pharmaceuticals has great importance, especially for pharmacokinetics, pharmacodynamics and epidemiological studies. In this review, liquid chromatographic methods developed in the last 10 years were summarized focusing on sample preparation and detection methods for HCQ and CQ determination in biological fluids and pharmaceutical preparations. It is hoped that this article could be helpful to facilitate the use of these drugs in clinical trials or drug research studies as it provides comprehensive information on the reported analytical methods.
© The Author(s) 2020. Published by Oxford University Press. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures
References
-
- WHO ; WHO Coronavirus Disease (COVID-19) Dashboard Overview. (2020) https://covid19.who.int/ (accessed July 25, 2020).
-
- Ferner R.E., Aronson J.K.; Chloroquine and Hydrochloroquine in covid 19; BMJ, (2020); 369: 1–2. - PubMed
-
- WHO ; WHO Q&A: Hydroxychloroquine and COVID-19 (2020). https://www.who.int/news-room/q-a-detail/q-a-hydroxychloroquine-and-covi... (accessed July 25, 2020).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources