Prognostic implications of dual tracer PET/CT: PSMA ligand and [18F]FDG PET/CT in patients undergoing [177Lu]PSMA radioligand therapy
- PMID: 33336265
- PMCID: PMC8113196
- DOI: 10.1007/s00259-020-05160-8
Prognostic implications of dual tracer PET/CT: PSMA ligand and [18F]FDG PET/CT in patients undergoing [177Lu]PSMA radioligand therapy
Abstract
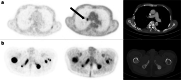
Background: Prostate-specific membrane antigen (PSMA)-targeted radioligand therapy (RLT) with 177Lu-labeled PSMA ligands has achieved remarkable results in advanced disease stages of metastatic castration-resistant prostate cancer (mCRPC). However, not all patients benefit from this therapy. Different treatment responses could be explained by tumor heterogeneity triggered by progression and the number of prior treatments. PSMA-negative lesions can be missed on PSMA ligand PET/CT, which subsequently results in an underestimation of tumor burden. Conversely, high FDG uptake may also be an indicator of tumor aggressiveness and thus a poor prognostic marker for response to RLT and overall survival (OS). The aim of this analysis was to investigate the prognostic value of combined PSMA ligand PET/CT and [18F]fluorodeoxyglucose (FDG) PET/CT for outcome prediction in patients undergoing RLT.
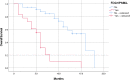
Materials and methods: This bicentric analysis included 54 patients with mCRPC who underwent both FDG and PSMA ligand PET/CT imaging before RLT. In all patients, the pattern of PSMA ligand and FDG uptake was visually assessed. Patients with at least one FDG-positive, but PSMA-negative (FDG+/PSMA-) lesions were compared to patients without any FDG+/PSMA- lesions. A log-rank analysis was used to assess the difference in OS between subgroups.
Results: Median OS was 11 ± 1.8 months (95% CI 7.4-14.6). A significantly lower OS (p < 0.001) was found in patients with at least one FDG+/PSMA- lesion at baseline PET/CTs (n = 18) with a median OS of 6.0 ± 0.5 months (95% CI: 5.0-7.0 months). In comparison, patients without any FDG+/PSMA- lesions (n = 36) had a median OS of 16.0 ± 2.5 months (95% CI: 11.2-20.8 months).
Conclusion: FDG+/PSMA- lesions are a negative predictor of overall survival in patients with mCRPC undergoing RLT. However, it remains to be determined if patients with FDG+/PSMA- lesions should be excluded from PSMA RLT.
Keywords: FDG; PET/CT; PSMA; Prostate cancer; Radioligand therapy.
Conflict of interest statement
The authors declare that they have no competing interests
Figures
Similar articles
-
Therapeutic efficacy, prognostic variables and clinical outcome of 177Lu-PSMA-617 PRLT in progressive mCRPC following multiple lines of treatment: prognostic implications of high FDG uptake on dual tracer PET-CT vis-à-vis Gleason score in such cohort.Br J Radiol. 2019 Dec;92(1104):20190380. doi: 10.1259/bjr.20190380. Epub 2019 Nov 1. Br J Radiol. 2019. PMID: 31600089 Free PMC article.
-
Response assessment using 68Ga-PSMA ligand PET in patients undergoing 177Lu-PSMA radioligand therapy for metastatic castration-resistant prostate cancer.Eur J Nucl Med Mol Imaging. 2019 May;46(5):1063-1072. doi: 10.1007/s00259-018-4236-4. Epub 2018 Dec 19. Eur J Nucl Med Mol Imaging. 2019. PMID: 30569186 Free PMC article.
-
Development of Discordant Hypermetabolic Prostate Cancer Lesions in the Course of [177Lu]PSMA Radioligand Therapy and Their Possible Influence on Patient Outcome.Cancers (Basel). 2021 Aug 25;13(17):4270. doi: 10.3390/cancers13174270. Cancers (Basel). 2021. PMID: 34503080 Free PMC article.
-
[177Lu]Lu-PSMA-Radioligand Therapy Efficacy Outcomes in Taxane-Naïve Versus Taxane-Treated Patients with Metastatic Castration-Resistant Prostate Cancer: A Systematic Review and Metaanalysis.J Nucl Med. 2023 Aug;64(8):1266-1271. doi: 10.2967/jnumed.123.265414. Epub 2023 May 11. J Nucl Med. 2023. PMID: 37169534
-
Factors predicting biochemical response and survival benefits following radioligand therapy with [177Lu]Lu-PSMA in metastatic castrate-resistant prostate cancer: a review.Eur J Nucl Med Mol Imaging. 2021 Nov;48(12):4028-4041. doi: 10.1007/s00259-021-05237-y. Epub 2021 Mar 6. Eur J Nucl Med Mol Imaging. 2021. PMID: 33677734 Free PMC article. Review.
Cited by
-
PSMA PET/CT in Castration-Resistant Prostate Cancer: Myth or Reality?J Clin Med. 2023 Nov 16;12(22):7130. doi: 10.3390/jcm12227130. J Clin Med. 2023. PMID: 38002742 Free PMC article. Review.
-
Management of Advanced Prostate Cancer in the Precision Oncology Era.Cancers (Basel). 2023 Apr 29;15(9):2552. doi: 10.3390/cancers15092552. Cancers (Basel). 2023. PMID: 37174018 Free PMC article. Review.
-
Extracting value from total-body PET/CT image data - the emerging role of artificial intelligence.Cancer Imaging. 2024 Apr 11;24(1):51. doi: 10.1186/s40644-024-00684-w. Cancer Imaging. 2024. PMID: 38605408 Free PMC article. Review.
-
[18F]FDG and [68Ga]Ga-FAPI-04 Imaging for Outcome Prediction in Patients with High-Grade Neuroendocrine Neoplasms.J Nucl Med. 2024 Dec 3;65(12):1899-1903. doi: 10.2967/jnumed.124.268288. J Nucl Med. 2024. PMID: 39477500 Free PMC article.
-
Biomarkers in Prostate-Specific Membrane Antigen Theranostics.Diagnostics (Basel). 2021 Jun 18;11(6):1108. doi: 10.3390/diagnostics11061108. Diagnostics (Basel). 2021. PMID: 34207069 Free PMC article. Review.
References
-
- Berthold DR, Pond GR, Soban F, de Wit R, Eisenberger M, Tannock IF. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer: updated survival in the TAX 327 study. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2008;26:242–245. doi: 10.1200/jco.2007.12.4008. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

