Plasmonic Temperature-Programmed Desorption
- PMID: 33337897
- PMCID: PMC7809689
- DOI: 10.1021/acs.nanolett.0c03733
Plasmonic Temperature-Programmed Desorption
Abstract
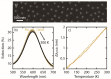
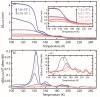
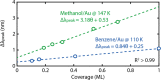
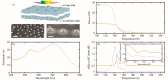
Temperature-programmed desorption (TPD) allows for the determination of the bonding strength and coverage of molecular mono- or multilayers on a surface and is widely used in surface science. In its traditional form using a mass spectrometric readout, this information is derived indirectly by analysis of resulting desorption peaks. This is problematic because the mass spectrometer signal not only originates from the sample surface but also potentially from other surfaces in the measurement chamber. As a complementary alternative, we introduce plasmonic TPD, which directly measures the surface coverage of molecular species adsorbed on metal nanoparticles at ultrahigh vacuum conditions. Using the examples of methanol and benzene on Au nanoparticle surfaces, the method can resolve all relevant features in the submonolayer and multilayer regimes. Furthermore, it enables the study of two types of nanoparticles simultaneously, which is challenging in a traditional TPD experiment, as we demonstrate specifically for Au and Ag.
Keywords: adsorption; metals; molecules; nanoparticles; plasmonic sensing; temperature-programmed desorption.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

References
-
- Habenschaden E.; Küppers J. Evaluation of Flash Desorption Spectra. Surf. Sci. 1984, 138 (1), L147–L150. 10.1016/0039-6028(84)90488-6. - DOI
-
- Gorte R. J. Temperature-Programmed Desorption for the Characterization of Oxide Catalysts. Catal. Today 1996, 28 (4), 405–414. 10.1016/S0920-5861(96)00249-0. - DOI
-
- McClure S. M.; Kim T. S.; Stiehl J. D.; Tanaka P. L.; Mullins C. B. Adsorption and Reaction of Nitric Oxide with Atomic Oxygen Covered Au(111). J. Phys. Chem. B 2004, 108 (46), 17952–17958. 10.1021/jp047335d. - DOI
-
- Gong J.; Flaherty D. W.; Ojifinni R. A.; White J. M.; Mullins C. B. Surface Chemistry of Methanol on Clean and Atomic Oxygen Pre-Covered Au(111). J. Phys. Chem. C 2008, 112 (14), 5501–5509. 10.1021/jp0763735. - DOI
-
- Murphy C. J.; Baggett A. W.; Miller D. P.; Simpson S.; Marcinkowski M. D.; Mattera M. F. G.; Pronschinske A.; Therrien A.; Liriano M. L.; Zurek E.; et al. Effect of BN/CC Isosterism on the Thermodynamics of Surface and Bulk Binding: 1,2-Dihydro-1,2-Azaborine vs Benzene. J. Phys. Chem. C 2015, 119 (26), 14624–14631. 10.1021/jp5126427. - DOI
LinkOut - more resources
Full Text Sources
Research Materials

