Immune response drives outcomes in prostate cancer: implications for immunotherapy
- PMID: 33338321
- PMCID: PMC8096785
- DOI: 10.1002/1878-0261.12887
Immune response drives outcomes in prostate cancer: implications for immunotherapy
Abstract
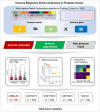
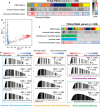
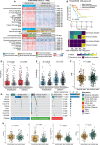
The heterogeneity of the immune microenvironment leads to different responses in immune checkpoint blockade therapy. We aimed to propose a robust molecular classification system to investigate the relevance of the immune microenvironment subtype and prognosis of prostate cancer patients, as well as the therapeutic response to immune checkpoint blockade therapy. A total of 1,557 prostate cancer patients were enrolled, including 69 real-world samples from our institute (titled the AHMU-PC cohort). The non-negative matrix factorization algorithm was employed to virtually microdissect patients. The immune enrichment was characterized by a high enrichment of T cell-, B cell-, NK cell-, and macrophage-associated signatures, by which patients were subclassified into nonimmune and immune classes. Subsequently, the immune class was dichotomized into immune-activated and immune-suppressed subtypes based on the stromal signature, represented by the activation of WNT/TGF-β, TGF-β1, and C-ECM signatures. Approximately 14.9% to 24.3% of patients belonged to the immune-activated subtype, which was associated with favorable recurrence-free survival outcomes. In addition, patients in the immune-activated subtype were predicted to benefit more from anti-PD-1/PD-L1 therapy. In conclusion, our study identifies a novel immune molecular classifier that is closely related to clinical prognosis and provides novel insights into immunotherapeutic strategies for prostate cancer patients.
Keywords: immune checkpoint blockade therapy; immune molecular subclassification system; immunotherapy; non-negative matrix factorization; prostate cancer.
© 2020 The Authors. Published by FEBS Press and John Wiley & Sons Ltd.
Conflict of interest statement
The authors have declared no conflicts of interest.
Figures


References
-
- Ferlay J, Colombet M, Soerjomataram I, Mathers C, Parkin DM, Pineros M, Znaor A & Bray F (2019) Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer 144, 1941–1953. - PubMed
-
- Omlin A, Pezaro C, Mukherji D, Mulick Cassidy A, Sandhu S, Bianchini D, Olmos D, Ferraldeschi R, Maier G, Thompson E et al, (2013) Improved survival in a cohort of trial participants with metastatic castration‐resistant prostate cancer demonstrates the need for updated prognostic nomograms. Eur Urol 64, 300–306. - PubMed
-
- Group P C T C (2000) Maximum androgen blockade in advanced prostate cancer: an overview of the randomised trials. Lancet 355, 1491–1498. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

