Bioactivity Potential of Marine Natural Products from Scleractinia-Associated Microbes and In Silico Anti-SARS-COV-2 Evaluation
- PMID: 33339096
- PMCID: PMC7765564
- DOI: 10.3390/md18120645
Bioactivity Potential of Marine Natural Products from Scleractinia-Associated Microbes and In Silico Anti-SARS-COV-2 Evaluation
Abstract
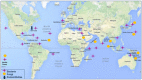
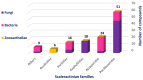
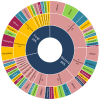
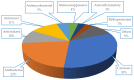
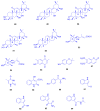
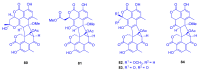
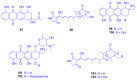
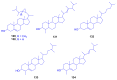
Marine organisms and their associated microbes are rich in diverse chemical leads. With the development of marine biotechnology, a considerable number of research activities are focused on marine bacteria and fungi-derived bioactive compounds. Marine bacteria and fungi are ranked on the top of the hierarchy of all organisms, as they are responsible for producing a wide range of bioactive secondary metabolites with possible pharmaceutical applications. Thus, they have the potential to provide future drugs against challenging diseases, such as cancer, a range of viral diseases, malaria, and inflammation. This review aims at describing the literature on secondary metabolites that have been obtained from Scleractinian-associated organisms including bacteria, fungi, and zooxanthellae, with full coverage of the period from 1982 to 2020, as well as illustrating their biological activities and structure activity relationship (SAR). Moreover, all these compounds were filtered based on ADME analysis to determine their physicochemical properties, and 15 compounds were selected. The selected compounds were virtually investigated for potential inhibition for SARS-CoV-2 targets using molecular docking studies. Promising potential results against SARS-CoV-2 RNA dependent RNA polymerase (RdRp) and methyltransferase (nsp16) are presented.
Keywords: ADME analysis; RNA-dependent RNA polymerase; SARS-CoV-2; Scleractinia; marine bacteria; marine fungi; marine natural products; methyltransferase; molecular docking; zooxanthellae.
Conflict of interest statement
The authors declare no conflict of interest.
Figures














References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

