Commercialized Blood-, Urinary- and Tissue-Based Biomarker Tests for Prostate Cancer Diagnosis and Prognosis
- PMID: 33339117
- PMCID: PMC7765473
- DOI: 10.3390/cancers12123790
Commercialized Blood-, Urinary- and Tissue-Based Biomarker Tests for Prostate Cancer Diagnosis and Prognosis
Abstract
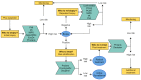
In the diagnosis and prognosis of prostate cancer (PCa), the serum prostate-specific antigen test is widely used but is associated with low specificity. Therefore, blood-, urinary- and tissue-based biomarker tests have been developed, intended to be used in the diagnostic and prognostic setting of PCa. This review provides an overview of commercially available biomarker tests developed to be used in several clinical stages of PCa management. In the diagnostic setting, the following tests can help selecting the right patients for initial and/or repeat biopsy: PHI, 4K, MiPS, SelectMDx, ExoDx, Proclarix, ConfirmMDx, PCA3 and PCMT. In the prognostic setting, the Prolaris, OncotypeDx and Decipher test can help in risk-stratification of patients regarding treatment decisions. Following, an overview is provided of the studies available comparing the performance of biomarker tests. However, only a small number of recently published head-to-head comparison studies are available. In contrast, recent research has focused on the use of biomarker tests in relation to the (complementary) use of multiparametric magnetic resonance imaging in PCa diagnosis.
Keywords: cancer biomarkers; liquid biopsies; prostate cancer; sPSA.
Conflict of interest statement
W.C.H.V., H.d.J. and W.J.G.M. are employees of MDxHealth. J.A.S. is a consultant for MDxHealth.
Figures
References
-
- Catalona W.J., Partin A.W., Sanda M.G., Wei J.T., Klee G.G., Bangma C.H., Slawin K.M., Marks L.S., Loeb S., Broyles D.L., et al. A multicenter study of [-2]pro-prostate specific antigen combined with prostate specific antigen and free prostate specific antigen for prostate cancer detection in the 2.0 to 10.0 ng/mL prostate specific antigen range. J. Urol. 2011;185:1650–1655. doi: 10.1016/j.juro.2010.12.032. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources