Zika M Oligopeptide ZAMP Confers Cell Death-Promoting Capability to a Soluble Tumor-Associated Antigen through Caspase-3/7 Activation
- PMID: 33339164
- PMCID: PMC7765671
- DOI: 10.3390/ijms21249578
Zika M Oligopeptide ZAMP Confers Cell Death-Promoting Capability to a Soluble Tumor-Associated Antigen through Caspase-3/7 Activation
Abstract
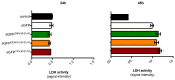
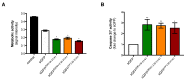
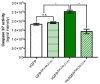
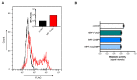
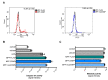
Mosquito-borne Zika virus (ZIKV) is an emerging flavivirus of medical concern associated with neurological disorders. ZIKV utilizes apoptosis as a mechanism of cell killing. The structural M protein may play a role in flavivirus-induced apoptosis. The death-promoting capability of M has been restricted to an oligopeptide representing the residues M-32/40. Here, we evaluated the apoptosis inducing ability of the residues M-31/41 of ZIKV. The ZIKV M oligopeptide was associated to a soluble form of GFP (sGFP) and the resulting sGFP-M31/41 construct was assessed in Huh7 cells. Expression of sGFP-M31/41 can trigger apoptosis in Huh7 cells through caspase-3/7 activation. The translocation of sGFP-M31/41 in the endoplasmic reticulum was a prerequisite for apoptosis induction. The residues M-33/35/38 may play a critical role in the death-promoting activity of sGFP-M31/41. The effect of ZIKV M oligopeptide defined as ZAMP (for Zika Apoptosis M Peptide) on expression of a tumor-associated antigen was assayed on megakaryocyte-potentiating factor (MPF). Expression of MPF-ZAMP construct resulted in caspase-associated apoptosis activation in A549 and Huh7 cells. ZIKV has been proposed as an oncolytic virus for cancer therapy. The ability of the Zika M oligopeptide to confer death-promoting capability to MPF opens up attractive perspectives for ZAMP as an innovative anticancer agent.
Keywords: M protein; Zika virus; anticancer agent; anticancer virotherapy; apoptotic cell death; arbovirus; caspase-3/7 activation; tumor cells; tumor-associated antigen; viral apoptosis inducer; viral oligopeptide.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Cao-Lormeau V.M., Blake A., Mons S., Lastere S., Roche C., Vanhomwegen J., Dub T., Baudouin L., Teissier A., Larre P., et al. Guillain-Barre Syndrome outbreak associated with Zika virus infection in French Polynesia: A case-control study. Lancet. 2016;387:1531–1539. doi: 10.1016/S0140-6736(16)00562-6. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

