S-adenosyl-l-homocysteine Hydrolase: A Structural Perspective on the Enzyme with Two Rossmann-Fold Domains
- PMID: 33339190
- PMCID: PMC7765604
- DOI: 10.3390/biom10121682
S-adenosyl-l-homocysteine Hydrolase: A Structural Perspective on the Enzyme with Two Rossmann-Fold Domains
Abstract
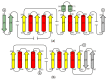
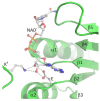
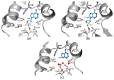
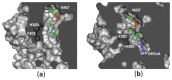
S-adenosyl-l-homocysteine hydrolase (SAHase) is a major regulator of cellular methylation reactions that occur in eukaryotic and prokaryotic organisms. SAHase activity is also a significant source of l-homocysteine and adenosine, two compounds involved in numerous vital, as well as pathological processes. Therefore, apart from cellular methylation, the enzyme may also influence other processes important for the physiology of particular organisms. Herein, presented is the structural characterization and comparison of SAHases of eukaryotic and prokaryotic origin, with an emphasis on the two principal domains of SAHase subunit based on the Rossmann motif. The first domain is involved in the binding of a substrate, e.g., S-adenosyl-l-homocysteine or adenosine and the second domain binds the NAD+ cofactor. Despite their structural similarity, the molecular interactions between an adenosine-based ligand molecule and macromolecular environment are different in each domain. As a consequence, significant differences in the conformation of d-ribofuranose rings of nucleoside and nucleotide ligands, especially those attached to adenosine moiety, are observed. On the other hand, the chemical nature of adenine ring recognition, as well as an orientation of the adenine ring around the N-glycosidic bond are of high similarity for the ligands bound in the substrate- and cofactor-binding domains.
Keywords: cellular methylation; nucleoside substrate; nucleotide cofactor; protein structure; protein-ligand interactions; structural enzymology.
Conflict of interest statement
The author declares no conflict of interest.
Figures


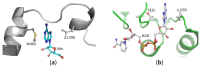

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

