Prebiotic Reaction Networks in Water
- PMID: 33339192
- PMCID: PMC7765580
- DOI: 10.3390/life10120352
Prebiotic Reaction Networks in Water
Abstract
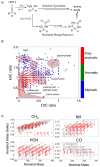
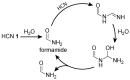
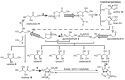
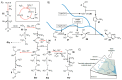
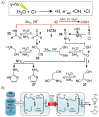
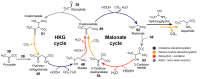
A prevailing strategy in origins of life studies is to explore how chemistry constrained by hypothetical prebiotic conditions could have led to molecules and system level processes proposed to be important for life's beginnings. This strategy has yielded model prebiotic reaction networks that elucidate pathways by which relevant compounds can be generated, in some cases, autocatalytically. These prebiotic reaction networks provide a rich platform for further understanding and development of emergent "life-like" behaviours. In this review, recent advances in experimental and analytical procedures associated with classical prebiotic reaction networks, like formose and Miller-Urey, as well as more recent ones are highlighted. Instead of polymeric networks, i.e., those based on nucleic acids or peptides, the focus is on small molecules. The future of prebiotic chemistry lies in better understanding the genuine complexity that can result from reaction networks and the construction of a centralised database of reactions useful for predicting potential network evolution is emphasised.
Keywords: RNA; autocatalysis; complexity; protometabolism; systems chemistry.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Plotkin G., Priami C. Transactions on Computational Systems Biology VI. Vol. 3380. Springer; Berlin/Heidelberg, Germany: 2005.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

