A clinical protocol of a comparative effectiveness trial of extended-release naltrexone versus extended-release buprenorphine with individuals leaving jail
- PMID: 33339633
- PMCID: PMC8898543
- DOI: 10.1016/j.jsat.2020.108241
A clinical protocol of a comparative effectiveness trial of extended-release naltrexone versus extended-release buprenorphine with individuals leaving jail
Abstract
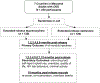
This study is a randomized, open label, controlled trial of extended-release buprenorphine (XR-B; BRIXADI™ formulation) versus extended-release naltrexone (XR-NTX) in Maryland jails. A 7-site, open-label, equivalence design will randomly assign 240 adults with a history of opioid use disorder (OUD), stratified by gender and jail, who are nearing release to one of two treatment arms: 1) XR-B in jail or 2) XR-NTX in jail, both followed by 6 monthly injections postrelease at a community treatment program. The primary aim is to determine the rate of pharmacotherapy adherence (number of monthly injections received) of XR-B compared to XR-NTX. The proposed study is innovative because it will be the first randomized clinical trial in the U.S. assessing the effectiveness of receiving XR-B vs. XR-NTX in county jails. The public health impact of the study will be highly significant and far-reaching because most individuals with OUD do not receive treatment while incarcerated, thereby substantially raising their likelihood of relapse to drug use, overdose death, and re-incarceration. Understanding how to expand acceptance of medications for OUD in jails, particularly extended-release medications, and supporting treatment engagement and medication adherence in transition to the community, has far-reaching implications for improving treatment access and success in this population.
Keywords: Extended-release buprenorphine; Extended-release naltrexone; Jail; Justice-involved; Medications for opioid use disorders.
Copyright © 2020 Elsevier Inc. All rights reserved.
Figures
References
-
- Aitkin M, Francis B, & Hinde J (2005). Statistical modelling in GLIM 4. Oxford University Press.
-
- Albayaty M, Linden M, Olsson H, Johnsson M, Strandgården K, & Tiberg F (2017). Pharmacokinetic evaluation of once-weekly and once-monthly buprenorphine subcutaneous injection depots (CAM2038) versus intravenous and sublingual buprenorphine in healthy volunteers under naltrexone blockade: An open-label Phase 1 study. Advances in Therapy, 34(2), 560–575. - PubMed
-
- Brinkley-Rubinstein L, Zaller N, Martino S, Cloud DH, McCauley E, Heise A, & Seal D (2018). Criminal justice continuum for opioid users at risk of overdose. Addictive Behaviors, 86, 104–110. - PubMed
-
- Bronson J, Stroop J, Zimmer S, & Berzofsky M (2017). Drug use, dependence, and abuse among state prisoners and jail inmates, 2007–2009. Washington, DC: United States Department of Justice, Bureau of Justice Statistics.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical