The diagnostic accuracy of isothermal nucleic acid point-of-care tests for human coronaviruses: A systematic review and meta-analysis
- PMID: 33339871
- PMCID: PMC7749114
- DOI: 10.1038/s41598-020-79237-7
The diagnostic accuracy of isothermal nucleic acid point-of-care tests for human coronaviruses: A systematic review and meta-analysis
Abstract
Many recent studies reported coronavirus point-of-care tests (POCTs) based on isothermal amplification. However, the performances of these tests have not been systematically evaluated. Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy was used as a guideline for conducting this systematic review. We searched peer-reviewed and preprint articles in PubMed, BioRxiv and MedRxiv up to 28 September 2020 to identify studies that provide data to calculate sensitivity, specificity and diagnostic odds ratio (DOR). Quality Assessment of Diagnostic Accuracy Studies 2 (QUADAS-2) was applied for assessing quality of included studies and Preferred Reporting Items for a Systematic Review and Meta-analysis of Diagnostic Test Accuracy Studies (PRISMA-DTA) was followed for reporting. We included 81 studies from 65 research articles on POCTs of SARS, MERS and COVID-19. Most studies had high risk of patient selection and index test bias but low risk in other domains. Diagnostic specificities were high (> 0.95) for included studies while sensitivities varied depending on type of assays and sample used. Most studies (n = 51) used reverse transcription loop-mediated isothermal amplification (RT-LAMP) to diagnose coronaviruses. RT-LAMP of RNA purified from COVID-19 patient samples had pooled sensitivity at 0.94 (95% CI: 0.90-0.96). RT-LAMP of crude samples had substantially lower sensitivity at 0.78 (95% CI: 0.65-0.87). Abbott ID Now performance was similar to RT-LAMP of crude samples. Diagnostic performances by CRISPR and RT-LAMP on purified RNA were similar. Other diagnostic platforms including RT- recombinase assisted amplification (RT-RAA) and SAMBA-II also offered high sensitivity (> 0.95). Future studies should focus on the use of un-bias patient cohorts, double-blinded index test and detection assays that do not require RNA extraction.
Conflict of interest statement
The authors declare no competing interests.
Figures
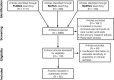



References
-
- Prompetchara E, Ketloy C, Palaga T. Immune responses in COVID-19 and potential vaccines: Lessons learned from SARS and MERS epidemic. Asian Pac. J. Allergy Immunol. 2020;38:1–9. - PubMed
-
- United Nation. https://www.un.org/sustainabledevelopment/blog/2020/04/covid-19-likely-t... (2020).
-
- Centers for Disease Control and Prevention. CDC 2019-Novel Coronavirus (2019-nCoV) Real-Time RT-PCR Diagnostic Panel. https://www.fda.gov/media/134922/download (2020).
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

