Cortical bone adaptation to a moderate level of mechanical loading in male Sost deficient mice
- PMID: 33339872
- PMCID: PMC7749116
- DOI: 10.1038/s41598-020-79098-0
Cortical bone adaptation to a moderate level of mechanical loading in male Sost deficient mice
Abstract
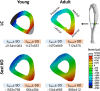
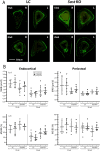
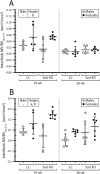
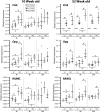
Loss-of-function mutations in the Sost gene lead to high bone mass phenotypes. Pharmacological inhibition of Sost/sclerostin provides a new drug strategy for treating osteoporosis. Questions remain as to how physical activity may affect bone mass under sclerostin inhibition and if that effect differs between males and females. We previously observed in female Sost knockout (KO) mice an enhanced cortical bone formation response to a moderate level of applied loading (900 με at the tibial midshaft). The purpose of the present study was to examine cortical bone adaptation to the same strain level applied to male Sost KO mice. Strain-matched in vivo compressive loading was applied to the tibiae of 10-, 26- and 52-week-old male Sost KO and littermate control (LC) mice. The effect of tibial loading on bone (re)modeling was measured by microCT, 3D time-lapse in vivo morphometry, 2D histomorphometry and gene expression analyses. As expected, Sost deficiency led to high cortical bone mass in 10- and 26-week-old male mice as a result of increased bone formation. However, the enhanced bone formation associated with Sost deficiency did not appear to diminish with skeletal maturation. An increase in bone resorption was observed with skeletal maturation in male LC and Sost KO mice. Two weeks of in vivo loading (900 με at the tibial midshaft) induced only a mild anabolic response in 10- and 26-week-old male mice, independent of Sost deficiency. A decrease in the Wnt inhibitor Dkk1 expression was observed 3 h after loading in 52-week-old Sost KO and LC mice, and an increase in Lef1 expression was observed 8 h after loading in 10-week-old Sost KO mice. The current results suggest that long-term inhibition of sclerostin in male mice does not influence the adaptive response of cortical bone to moderate levels of loading. In contrast with our previous strain-matched study in females showing enhanced bone responses with Sost ablation, these results in males indicate that the influence of Sost deficiency on the cortical bone formation response to a moderate level of loading differs between males and females. Clinical studies examining antibodies to inhibit sclerostin may need to consider that the efficacy of additional physical activity regimens may be sex dependent.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

