Toll-like receptor signalling in B cells during systemic lupus erythematosus
- PMID: 33339987
- PMCID: PMC7747191
- DOI: 10.1038/s41584-020-00544-4
Toll-like receptor signalling in B cells during systemic lupus erythematosus
Abstract
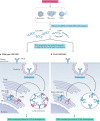
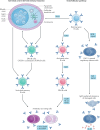
B lymphocytes have a central role in autoimmune diseases, which are often defined by specific autoantibody patterns and feature a loss of B cell tolerance. A prototypic disease associated with B cell hyperactivity is systemic lupus erythematosus (SLE). In patients with SLE, the loss of B cell tolerance to autoantigens is controlled in a cell-intrinsic manner by Toll-like receptors (TLRs), which sense nucleic acids in endosomes. TLR7 drives the extrafollicular B cell response and the germinal centre reaction that are involved in autoantibody production and disease pathogenesis. Surprisingly, TLR9 seems to protect against SLE, even though it is required for the production of autoantibodies recognizing double-stranded DNA-associated antigens, which are abundant in SLE and are a hallmark of this disease. The protective function of TLR9 is at least partly mediated by its capacity to limit the stimulatory activity of TLR7. The roles of TLR7 and TLR9 in the effector function of B cells in lupus-like disease and in patients with SLE, and the unique features of TLR signalling in B cells, suggest that targeting TLR signalling in SLE might be therapeutically beneficial.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Crickx E, Weill JC, Reynaud CA, Mahevas M. Anti-CD20-mediated B-cell depletion in autoimmune diseases: successes, failures and future perspectives. Kidney Int. 2020;97:885–893. - PubMed
-
- Shen P, Fillatreau S. Antibody-independent functions of B cells: a focus on cytokines. Nat. Rev. Immunol. 2015;15:441–451. - PubMed
-
- Davis MLR, et al. Associations of toll-like receptor (TLR)-4 single nucleotide polymorphisms and rheumatoid arthritis disease progression: an observational cohort study. Int. Immunopharmacol. 2015;24:346–352. - PubMed
-
- Ruprecht CR, Lanzavecchia A. Toll-like receptor stimulation as a third signal required for activation of human naive B cells. Eur. J. Immunol. 2006;36:810–816. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

