Responses to acute infection with SARS-CoV-2 in the lungs of rhesus macaques, baboons and marmosets
- PMID: 33340034
- PMCID: PMC7890948
- DOI: 10.1038/s41564-020-00841-4
Responses to acute infection with SARS-CoV-2 in the lungs of rhesus macaques, baboons and marmosets
Erratum in
-
Author Correction: Responses to acute infection with SARS-CoV-2 in the lungs of rhesus macaques, baboons and marmosets.Nat Microbiol. 2021 Mar;6(3):413. doi: 10.1038/s41564-021-00867-2. Nat Microbiol. 2021. PMID: 33462437 Free PMC article. No abstract available.
Abstract
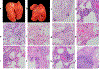
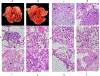
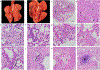
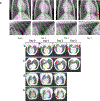
Non-human primate models will expedite therapeutics and vaccines for coronavirus disease 2019 (COVID-19) to clinical trials. Here, we compare acute severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in young and old rhesus macaques, baboons and old marmosets. Macaques had clinical signs of viral infection, mild to moderate pneumonitis and extra-pulmonary pathologies, and both age groups recovered in two weeks. Baboons had prolonged viral RNA shedding and substantially more lung inflammation compared with macaques. Inflammation in bronchoalveolar lavage was increased in old versus young baboons. Using techniques including computed tomography imaging, immunophenotyping, and alveolar/peripheral cytokine response and immunohistochemical analyses, we delineated cellular immune responses to SARS-CoV-2 infection in macaque and baboon lungs, including innate and adaptive immune cells and a prominent type-I interferon response. Macaques developed T-cell memory phenotypes/responses and bystander cytokine production. Old macaques had lower titres of SARS-CoV-2-specific IgG antibody levels compared with young macaques. Acute respiratory distress in macaques and baboons recapitulates the progression of COVID-19 in humans, making them suitable as models to test vaccines and therapies.
Figures
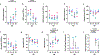


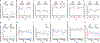

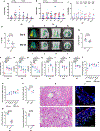
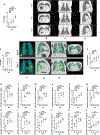



Comment in
-
More monkey models for studying COVID-19.Lab Anim (NY). 2021 Feb;50(2):44. doi: 10.1038/s41684-021-00717-y. Lab Anim (NY). 2021. PMID: 33505014 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
- T32 AG021890/AG/NIA NIH HHS/United States
- R01 AI134236/AI/NIAID NIH HHS/United States
- P51OD011133/U.S. Department of Health & Human Services | NIH | NIH Office of the Director (OD)/International
- R01AI134236/U.S. Department of Health & Human Services | NIH | National Institute of Allergy and Infectious Diseases (NIAID)/International
- P30 AG044271/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

