Autophagy and the endolysosomal system in presynaptic function
- PMID: 33340068
- PMCID: PMC8004491
- DOI: 10.1007/s00018-020-03722-5
Autophagy and the endolysosomal system in presynaptic function
Abstract
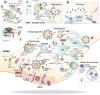
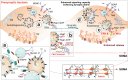
The complex morphology of neurons, the specific requirements of synaptic neurotransmission and the accompanying metabolic demands create a unique challenge for proteostasis. The main machineries for neuronal protein synthesis and degradation are localized in the soma, while synaptic junctions are found at vast distances from the cell body. Sophisticated mechanisms must, therefore, ensure efficient delivery of newly synthesized proteins and removal of faulty proteins. These requirements are exacerbated at presynaptic sites, where the demands for protein turnover are especially high due to synaptic vesicle release and recycling that induces protein damage in an intricate molecular machinery, and where replacement of material is hampered by the extreme length of the axon. In this review, we will discuss the contribution of the two major pathways in place, autophagy and the endolysosomal system, to presynaptic protein turnover and presynaptic function. Although clearly different in their biogenesis, both pathways are characterized by cargo collection and transport into distinct membrane-bound organelles that eventually fuse with lysosomes for cargo degradation. We summarize the available evidence with regard to their degradative function, their regulation by presynaptic machinery and the cargo for each pathway. Finally, we will discuss the interplay of both pathways in neurons and very recent findings that suggest non-canonical functions of degradative organelles in synaptic signalling and plasticity.
Keywords: Autophagy; Axonal boutons; Endolysosomal system; Proteostasis; Synaptic plasticity.
Conflict of interest statement
All authors have read the manuscript and have no competing interests.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

